The Ultimate Guide to Clinical Research Monitoring
Clinical research monitoring plays a crucial role in the success of clinical trials, encompassing diverse activities to guarantee the safety and precision of collected data. The execution of clinical trials must adhere to regulatory standards, prioritize the protection of human study participants, and minimize potential health risks. Monitoring activities include auditing study sites, assessing data accuracy and completeness, protocol and amendment reviews, scrutiny of case report forms (CRFs), identification of deviations from standard operating procedures (SOPs) or protocols, management of corrective action plans (CAPs), safety report follow-ups, and tracking progress against enrollment goals.
For those interested in becoming involved in clinical trial management and oversight, the Advanced Clinical Research Project Manager Certification offers extensive training in these key areas.
Beyond data quality assessment, clinical research monitoring ensures compliance with regulatory standards such as GCP (Good Clinical Practices), ICH (International Conference on Harmonization), FDA regulations, and local laws. Those looking to deepen their understanding of these standards might find the ICH-GCP course particularly beneficial. Ongoing monitoring throughout a study, coupled with potential audits by sponsors or regulatory authorities, contributes to the accuracy, reliability, and applicability of clinical trial results for informed medical decisions.
Steps to Clinical Monitoring:
Craft a Robust Monitoring Strategy: Develop a thorough monitoring plan encompassing essential elements. This includes specifying the types of monitoring activities, setting the frequency of monitoring visits, outlining data collection methods, and establishing clear criteria for acceptable performance.
Aspiring Clinical Research Coordinators can enhance their strategy formulation skills through the Clinical Research Coordinator course.
Create Effective Documentation: Develop monitoring tools tailored to the protocol, including forms for recording information from site visits, source documents, data collection instruments, and case report forms (CRF). Additionally, establish a Monitoring Log or Tracking System to enhance accountability for study activities.
For detailed training on documentation practices, consider the Clinical Trials Assistant Training.
Conducting Monitors' Visits: Depending on the trial's complexity and regulatory mandates, execute pre-study qualification visits (PSQV), pre-initiation visits (PIV), initiation visits (IVs), periodic monitoring visits (PMV), and close-out visits (COV). Throughout each visit, uphold good clinical practice standards by thoroughly reviewing source documents and data collection instruments. Scrutinize patient enrollment logs for accuracy, noting any discrepancies in the comprehensive visit report.
The CRA course provides in-depth training for those looking to conduct these critical visits.
Reporting Findings: Create comprehensive yet succinct reports after each monitor's visit, offering clear recommendations for corrective actions as needed. Provide professional feedback to investigators, highlighting their performance. Identify and address any noncompliance with protocol requirements or regulations, suggesting training or educational sessions when necessary. Track all follow-up activities related to corrective actions taken in response to monitor's visit findings. Ensure the completion of essential documentation before closing out a specific study site.
Those interested in the oversight of safety reporting might explore the Pharmacovigilance Certification.
Ensuring Quality Assurance: Validate the accuracy of tracking systems employed by monitors during their visits. Assess the risks linked to identified deficiencies throughout the monitoring process. Conduct regular internal audits/assessments to guarantee compliance with established SOPs/guidelines pertaining to clinical research monitoring activities. Implement preventive measures based on audit/assessment results to enhance internal quality system processes.
Professionals aiming for a specialized role in this field might consider the Medical Monitor Certification.
Types of Clinical Trial Monitoring
Onsite Monitoring: Onsite monitoring, considered the "gold standard," entails a monitor's presence at a study site throughout the trial. The monitor reviews source documentation, including patient records, lab results, and investigational product dispensing logs, ensuring accuracy and compliance with study protocols and good clinical practices (GCP). Staff interviews verify proper adherence to trial procedures.
Centralized or Remote Monitoring in Clinical Trials: Centralized or remote monitoring allows sponsors to conduct clinical research monitoring without onsite visits. Leveraging technology like web portals and video conferencing, monitors remotely review data from multiple sites simultaneously. This method facilitates quick issue identification. Moreover, it enables proactive risk assessment before onsite visits, enhancing the efficiency of the monitoring process.
Types of Clinical Research Monitoring: Clinical research monitoring is a critical process that evaluates the quality and integrity of clinical trial data, ensuring adherence to regulatory requirements. Three primary methods are employed: onsite monitoring, centralized or remote monitoring, and risk-based approaches.
4. Risk-Based Approaches (2024): Embracing the advancements of 2024, risk-based approaches now leverage cutting-edge data analytics tools like advanced descriptive statistics and predictive algorithms. These tools identify potential trends or outliers in clinical trial data, signaling an increased risk of noncompliance with Good Clinical Practices (GCPs) or other regulations. Technology-driven approaches enable sponsors to detect issues earlier in a trial, allowing timely corrective action to prevent complications.
5. Benefits of Clinical Research Monitoring (2024): In the ever-evolving landscape of clinical research, effective monitoring strategies play a pivotal role in ensuring trials are conducted ethically, safely, and in accordance with protocol standards. Aligned with timelines agreed upon with regulatory authorities and budget constraints set by sponsors/CROs/investigators, these strategies provide invaluable insights. Acting as independent third parties, clinical research monitors offer objective perspectives across multiple sites, minimizing biases from investigators or personnel with vested interests.
Furthermore, contemporary monitoring ensures patient safety by overseeing the administration of drugs or medical devices and maintaining confidentiality throughout the study. Robust monitoring protocols also prove instrumental in reducing costs associated with potential delays, preventing errors throughout the trial duration, from pre-study startup to post-closeout when all enrolled patients have completed their participation.
Clinical Research Monitoring Guide
1. Mastering Clinical Research Monitoring in 2024:
Dive into the core of clinical research monitoring, a vital aspect of the research process ensuring both safety and result accuracy. Regular assessments of study sites verify proper data collection in adherence to ethical standards, legal requirements, and the latest Good Clinical Practice (GCP) guidelines.
2. Demystifying Monitored Study Types:
In the SEO landscape of 2024, clinical research monitoring extends beyond clinical trials to encompass observational studies, epidemiologic studies, and public health surveys. Understanding the specific study type being monitored is crucial for ensuring the correct procedures are implemented.
3. Navigating Study Site Monitoring:
Stay current with 2024 SEO standards by comprehending the intricacies of clinical research monitoring. The primary objective is meticulous confirmation that both protocol and informed consent forms are followed at each site. This involves in-depth reviews of relevant documents such as case report forms (CRFs), source documentation (e.g., physician notes), internal audit reports, and external quality assurance reports. Compliance with GCP guidelines during site visits or remote reviews, coupled with interviews assessing data collection and reporting processes, enhances the monitoring process.
4. Grasping Regulatory Requirements in 2024:
Beyond GCP guidelines, the SEO-friendly approach for 2024 emphasizes an awareness of applicable regulations from local governments or institutions. Adherence to these regulations is vital for compliance with laws related to clinical research monitoring activities.
5. Crafting an Advanced Monitoring Plan:
Elevate your monitoring plan in 2024 with a detailed timeline for site visits, specific focuses (e.g., patient enrollment/randomization, adverse event management), and strategies for auditing/reviewing generated data. Incorporate measures to control data collection risks, enabling early issue identification, aligning with SEO standards and ensuring a smooth study process.
Clinical Research Monitor Job
A Clinical Research Monitor plays a crucial role in ensuring the ethical and safe conduct of clinical trials while maintaining compliance with established standards. The primary focus is safeguarding the rights, safety, and well-being of human subjects participating in the trials. Responsibilities encompass a wide range of activities, including protocol development, coordination of study start-up, site visits, monitoring data accuracy and completeness, auditing files for regulatory compliance, managing investigator queries, preparing visit reports, reviewing protocol updates, resolving issues identified through audits, offering technical guidance to sites on protocol implementation, and escalating complex issues or potential risks.
Clinical Research Monitor Salary
The salary for this position varies based on factors such as education, experience, and geographical location. Entry-level positions may start at around $60,000 per year, while experienced professionals can earn up to approximately $90,000 per year. In addition to salary, many employers provide benefits such as paid vacation days, health insurance plans, and retirement packages.
Resources for Clinical Research Monitoring
1. National Institutes of Health (NIH): Clinical Research Monitoring
This link provides information on NIH's guidelines for monitoring clinical research, which include topics such as the roles and responsibilities of the investigator, data safety monitoring boards, and protocols for reporting unanticipated problems and adverse events.
2. National Institutes of Health (NIH): Guide to Clinical Research Monitoring
This comprehensive guide walks readers through all aspects of clinical research monitoring, including topics such as study design, randomization strategies, regulatory compliance requirements, data management, monitoring plans and reports, quality improvement initiatives, and safety assessments.
3. US Food and Drug Administration (FDA): Guidelines for Clinical Trials Monitoring
This resource from the FDA outlines the importance of effective monitoring in clinical trials and provides an overview of the different roles within a clinical trial as well as details about essential elements for implementation of an effective monitoring strategy such as risk assessments and adverse event tracking.
4. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH)
ICH has developed standards that provide a set of harmonized technical requirements for clinical trials conducted across countries in the European Union (EU), Japan, and US with an emphasis on quality assurance and safety monitoring during trials.
5. Association of Clinical Research Professionals (ACRP)
ACRP's guidelines provide best practice recommendations for conducting clinical research studies in accordance with applicable regulations and standards to ensure patient safety monitoring during studies as well as data integrity throughout the process from start to finish.
6. Pharmaceutical Research & Manufacturers of America (PhRMA)
The PhRMA guidelines provide an overview of expectations around clinical research activities with respect to ethics, data integrity, safety reporting, resource allocation and more. It defines roles and responsibilities of all those involved in overseeing a clinical trial such as a Clinical Research Monitor or CRA who has primary responsibility for ensuring that the protocol is implemented correctly throughout a study’s duration
Clinical Research Monitoring Review
1. What is the main purpose of clinical research monitoring?
A) To ensure that a research study is conducted in accordance with applicable regulations and ethical standards
B) To ensure that data collected during a research study is accurate and reliable
C) To evaluate the safety of participants enrolled in a research trial
D) To oversee the financial management of a research project
Answer: A) To ensure that a research study is conducted in accordance with applicable regulations and ethical standards. Clinical Research Monitors are responsible for ensuring compliance with Good Clinical Practice guidelines, protecting participant privacy, verifying data accuracy, and evaluating protocol deviations. In addition, they may also be involved in reviewing participant eligibility requirements, conducting site assessments, providing training to investigators and staff on proper study procedures, as well as monitoring progress towards completion of all requirements of the study.
2. What type of individuals typically serve as clinical research monitors?
A) Physicians
B) Nurses
C) Regulatory specialists
D) All of the above
Answer: D) All of the above. Clinical Research Monitors can come from various backgrounds such as medical doctors (MDs), nurses (RNs), pharmacists (RPhs), regulatory specialists (e.g., Regulatory Affairs Professionals or Paralegals), or biostatisticians/data analysts who have experience in clinical trials and understand local regulations related to human subject protection. Each monitor has specific job duties depending on their education and experience, such as assessing compliance with regulatory guidance or analyzing data sets for accuracy, completeness, integrity, or validity.
3. What kind of activities do clinical research monitors need to perform?
A) Protocol reviews or verifications
B) Ensuring appropriate documentation completion
C) Site visits to observe investigator conduct
D )All of the above
Answer: D )All of the above. Clinical Research Monitors need to perform several activities including protocol reviews or verifications; ensuring appropriate documentation completion; site visits to observe investigator conduct; liaising between sponsors and sites; assisting with resolving issues associated with adverse events; reviewing case report forms for completeness, accuracy, consistency and correctness; evaluating subject safety throughout enrollment process;and writing reports detailing their findings at each visit.
4. What is one benefit gained from having an effective Clinical Research Monitor on-site? A) Reduced risk for legal liability stemming from negligence
B) Improved protocol adherence by investigators
C) Increased patient engagement during trial period
D )All of the above
Answer: D) All of the above . An effective Clinical Research Monitor encompasses several benefits such as reduced risk for legal liability stemming from negligence due to thorough oversight and accurate record keeping; improved protocol adherence by investigators through continued communication between sponsor representatives and researchers on-site regarding best practices; increased patient engagement during trial period due to more detailed explanations about potential risks/benefits offered by having monitor on-site ; and improved efficiency when dealing with complex protocols that require multiple levelsof oversight due to familiarity with protocol specifics which decreases time spent troubleshooting errors or unclear instructions..
5. How often should Clinical Research Monitors visit a particular site?
A) Weekly B) Biweekly C) Monthly D) Quarterly
Answer: C) Monthly . It is recommended that Clinical Research Monitors visit sites at least once per month in order to maintain active surveillance over ongoing studies at each location while also providing timely feedback regarding any issues discovered while on-site visits are taking place within a shorter timeframe if needed based upon changes made midstream or other unanticipated circumstances which might require immediate attention by sponsor personnel.
Medical Monitoring in Clinical Research - Non Clinical Physician Jobs
What Is medical monitor in Clinical Research?
A Full Overview and Guide to Becoming a Physician Medical Monitor
Considered a “Hidden Gem” for Non Clinical Physician Jobs
Clinical trials are the bedrock of medical progress, meticulously evaluating the efficacy and safety of novel drugs and treatments. Within this vital process, medical monitors (MMs) play an indispensable role. These physician-experts oversee critical aspects of the trial, guaranteeing both patient well-being and the scientific integrity of the research. For those interested in this role, consider our Medical Monitor Certification.
Distinct Expertise: Physician-Level Oversight Makes the Difference
While clinical research associates (CRAs) ensure protocol adherence and accurate adverse event (AE) reporting at trial sites, medical monitors bring a physician's perspective. They leverage their medical knowledge to:
Guide Protocol Development: Medical monitors advise on designing protocols that prioritize patient safety and align with current best practices as outlined by the National Institutes of Health (NIH) in their guidance document, "Clinical Trial Design Considerations for Safety" NIH Guidance. For those looking to further understand protocol development, consider our ICH-GCP course.
Evaluate Safety Concerns: Throughout the trial, they assess and address patient safety issues that may arise, referencing the Food and Drug Administration's (FDA) "Guidance for Investigators for Conduct of Human Clinical Trials" FDA Guidance for best practices.
Make Unblinding Decisions: In rare cases of severe AEs, medical monitors determine if unblinding is necessary for effective intervention, following the European Medicines Agency's (EMA) ICH Guideline E6(R2) on Good Clinical Practice EMA Guidance. Aspiring professionals can deepen their understanding with our Advanced Clinical Research Project Manager Certification.
Beyond Oversight: Fostering Effective Collaboration
Similar to CRAs, medical monitors act as liaisons between sponsors and trial sites. They review and ensure proper coding and reporting of AEs, guaranteeing data accuracy and adherence to regulatory guidelines set forth by organizations like the International Council for Harmonisation of Good Clinical Practice (ICH-GCP). For further training in these responsibilities, explore our Clinical Research Coordinator course and Pharmacovigilance Certification.
The Multifaceted Role of the Medical Monitor
The complexities of clinical research in 2024 demand a multifaceted role for medical monitors. Their responsibilities encompass:
Protocol Review and Design: Scrutinizing protocols to prioritize patient safety and ensure alignment with best practices. Consider our CRA course for detailed insights into this role.
Safety Oversight: Continuously monitoring patient well-being throughout the trial and addressing any safety concerns.
Data Analysis and Interpretation: Collaborating with the research team to analyze and interpret trial data effectively.
Regulatory Compliance: Ensuring the trial adheres to all relevant regulatory requirements.
Communication and Collaboration: Acting as a bridge between sponsors, investigators, and research teams, fostering clear communication and collaboration.
Specialist Services for Streamlined Trials
Contract research organizations (CROs) and sponsors often partner with specialist companies like C3i Solutions and GeorgeClinical to leverage their expertise in providing medical monitor services. For those aspiring to excel in assisting clinical trials, our Clinical Trials Assistant Training offers comprehensive insights.
Additionally, for those interested in reaching the pinnacle of research oversight, consider the Advanced Principal Investigator Physician Certification.
Essential Resources
National Institutes of Health (NIH): Role of Medical Monitors in Safety Oversight https://www.niaid.nih.gov/sites/default/files/medicalmonitor.pdf
Explore Our Clinical Research Training Programs:
Key Distinctions: Medical Monitor vs. Other Clinical Trial Roles
It's crucial to distinguish the medical monitor from other vital clinical trial personnel. Here's a quick breakdown:
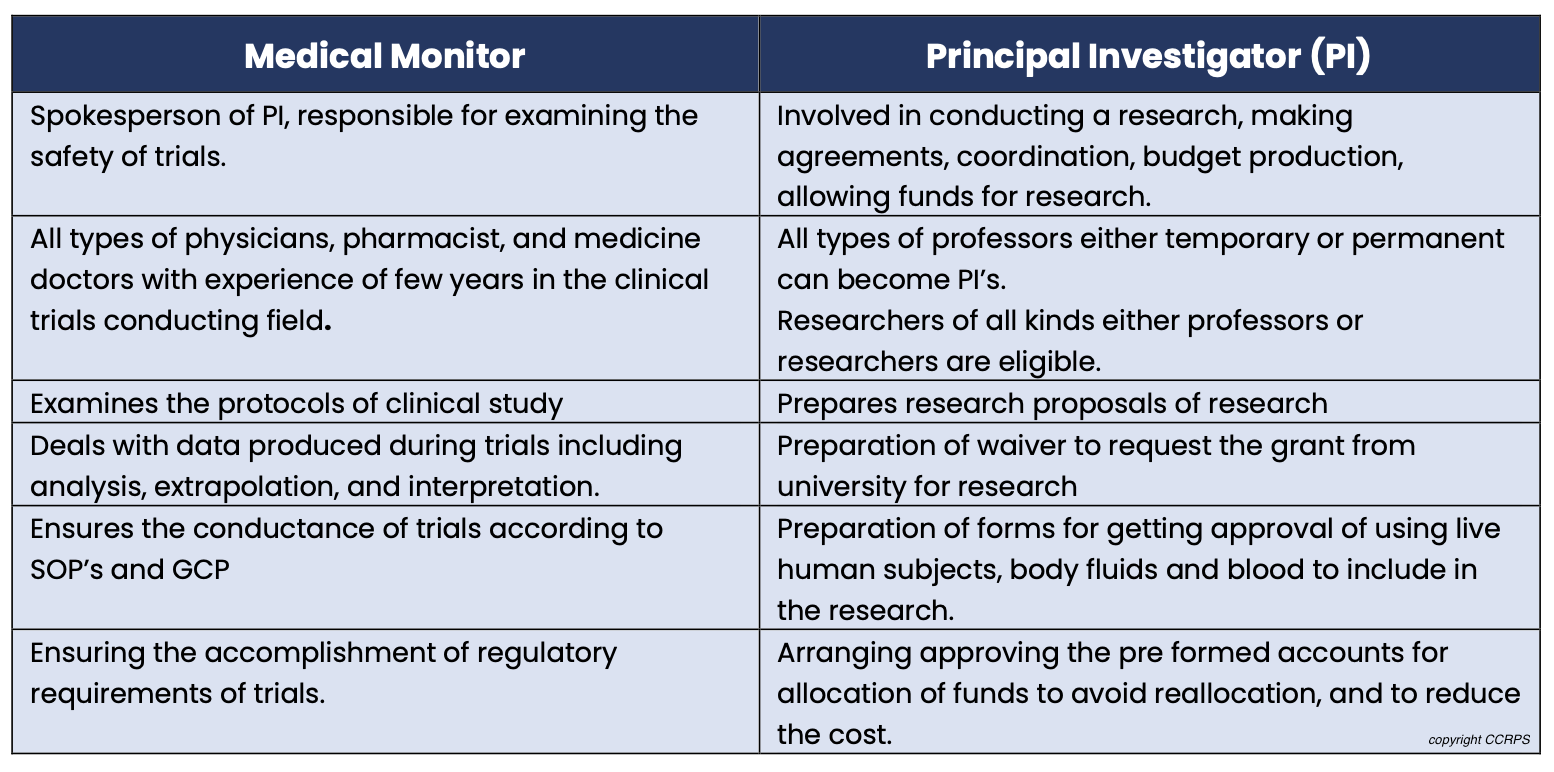
Medical Monitor vs. Principal Investigator (PI): PIs lead trials at a specific site, while medical monitors offer broader oversight across multiple trials and sites.
Medical Monitor vs. Clinical Research Associate (CRA): CRAs focus on protocol adherence and data collection at a single site, whereas medical monitors provide physician-level expertise for broader trial aspects.
Understanding Medical Monitoring According to E6-GCP
The E6 Good Clinical Practice (GCP) guidelines define a medical monitor as a person responsible for supervising the clinical trial process. They ensure that protocols, standard operating procedures (SOPs), Good Manufacturing Practices (GMP), and regulatory requirements are all followed according to established standards as outlined in the European Medicines Agency's (EMA) ICH Guideline E6(R2) on Good Clinical Practice https://www.ema.europa.eu/en/human-regulatory-overview/research-and-development/compliance-research-and-development/good-clinical-practice
Alternative Terminologies for Medical Monitor
It's important to note that medical monitor is not the only term used for this role. Here are some alternatives:
Clinical research associate (CRA)
Site manager
Senior CRA
Clinical trial assistant (CTA)
However, clinical research associate (CRA) is the most frequently used alternative. Be aware that while there may be some overlap in responsibilities, CRAs
Difference between a Medical Monitor and Principal Investigator
What is a medical monitor in clinical research
Salary of a medical monitor
Salary of medical monitor varies due to different factors including education, certifications, additional skills, number of experience years in profession.
The average salary of a medical monitor is 155,000$ and salary usually ranges from 87,000$- 398,000 (Ziprecruiter).
The national average for CRA is $65-119k which is different than medical monitors working in the pharmaceutical companies.
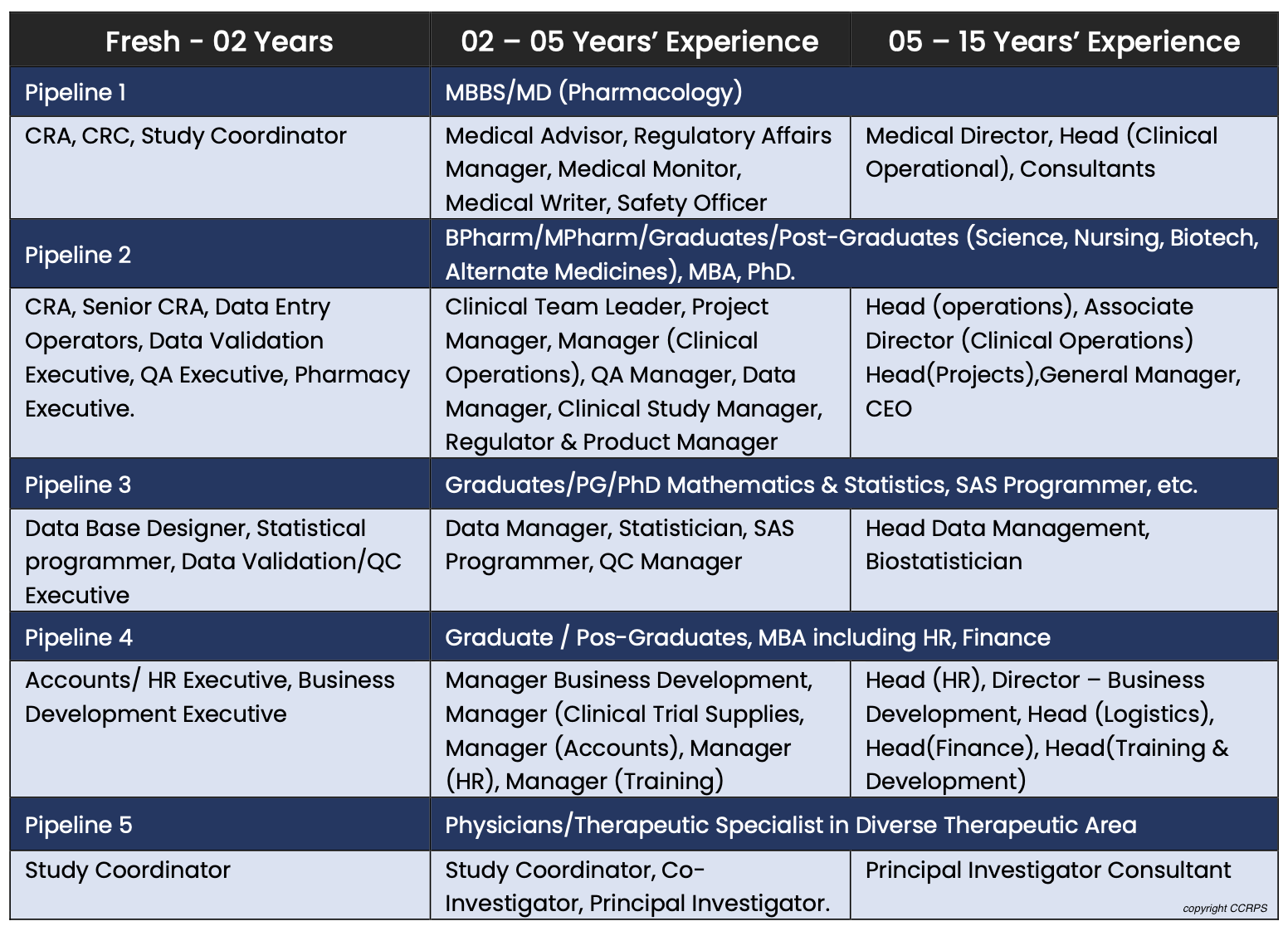
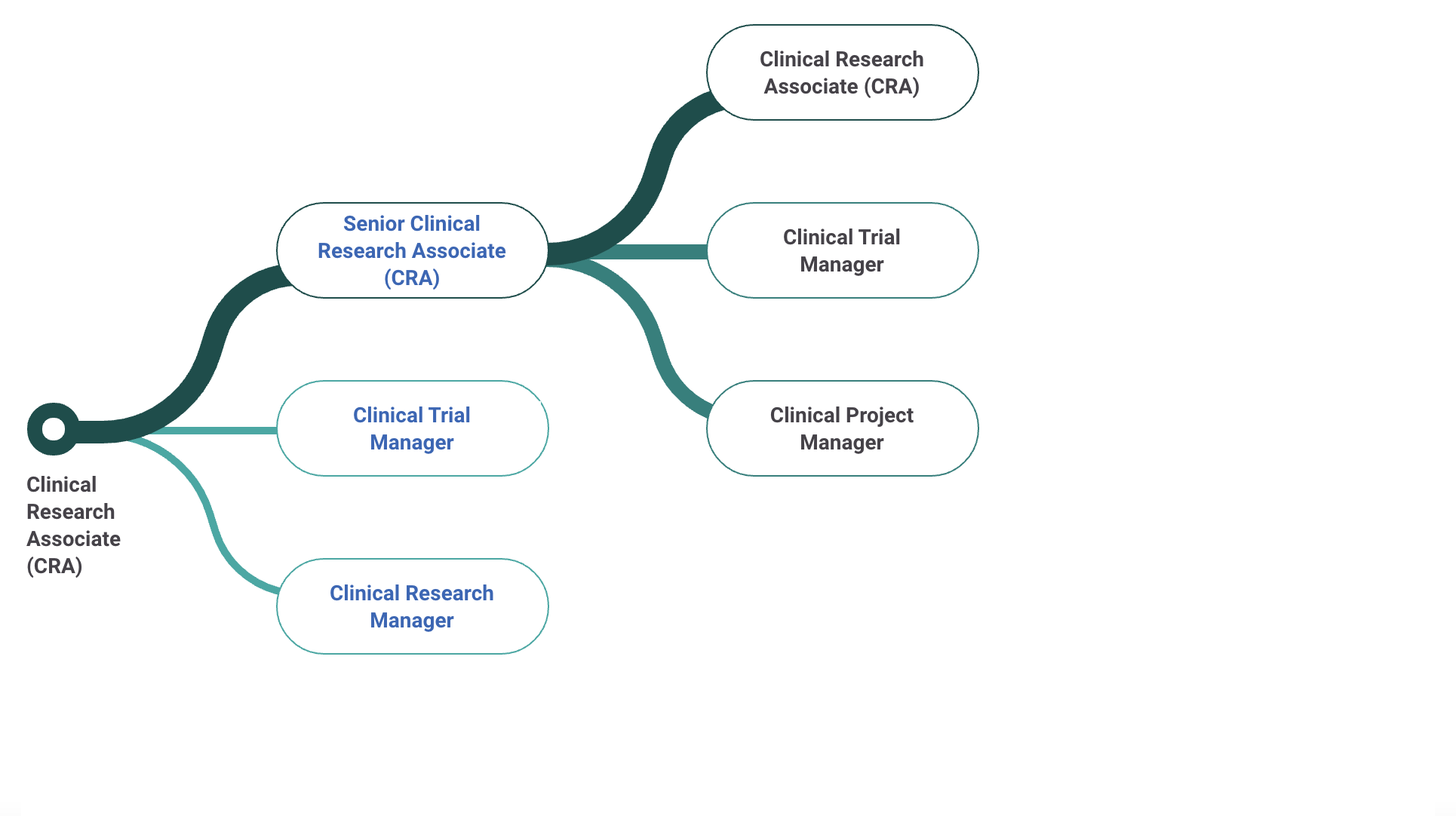
There are so many opportunities in the clinical research industry. At the site level, some are study coordinator, principal investigator, sub investigator, research assistant. At the CRO level there are CRA's, CTA's, CMA's, medical monitors, project managers. At a Sponsor level there are dozens more. What is good about many of these, is one's ability to "level up" over time into more desirable and higher paying positions.
Skills and qualifications required to be a medical monitor:
Medical degree with strong leadership skills
Direct experience in the pharmaceutical industry (i.e. Clinical Research Associate; preferred; although with CCRPS Medical Monitor Certification you are qualified to work as both a CRA and MM)
The preferred countries for the experience are US and EU
Knowledge of both local and Global regulatory requirements as well as the
knowledge of local and Global GCP
Skillful in converting input into regulatory documentation.
Strong communication skills to deal with stakeholders either internal or external.
Strong writing and presentation skills.
Skillful to deal with audience especially medical and scientific community.
Relevant work experience in medical monitoring and/or pharmacovigilance
and/or drug safety experience in a CRO, pharmaceutical, or clinical trial environment required.
Must have appropriate understanding about the International conference on Harmonization (ICH), GCP guidelines.
Must have appropriate knowledge of research, clinical trials and clinical terminologies.
Having strong decision-making power to deal with the trials.
Proficiency in English (written and verbal) required.
Capable of building and maintaining the trust of clients.
You can showcase and gain the knowledge you need to work as a MM with CCRPS’s Medical Monitor certification which trains you to a Senior Monitor’s level of knowledge to allow for easy promotion in the field. You do need experience before getting promoted so we suggest getting an internship while studying for the course prior to applying for Medical Monitor or CRA jobs!
Individuals who should try to be a medical monitor include:
Typically this is a fully qualified physician (MD, MBBS, IMG, FMG)
Sometimes residency in the work-specific department or in internal medicine is helpful (whether international or US-completed)
In the US it is also permitted to also use a PharmD (pharmacist) as a medical monitor
Role and responsibilities of a Medical monitor:
At the clinical trials site, role of the monitor depends upon the experience of the monitor as well as the phase of the ongoing trial. A monitor is a competent person performing differant responsibilities and activities at different sites in accordance with the phases of trials. Some of these responsibilities are:
While discussing and presenting the facts and initiating a trial with investigator a monitor must become a mentor and counsellor.
Responsible for developing devices and conducting research on drugs in improving the quality of life of patients.
Providing monitoring reports, the guidance about further actions, lacking in the study, elaborating findings to improve compliance.
Dealing with the recruitment of the research patients and their retention or exclusion for obtaining quality findings.
Situation handling is the main responsibility of the monitor to safely resolve the issue by troubleshooting the problem.
Planning of travelling costs and schedules are also maintained by the monitor.
A monitor works as a detector when it comes to decide a site for trial and visiting the
sites.
Effective communication and convincing ability while dealing with different levels of
the people is the major skill and responsibility of the medical monitor.
Developing regulatory and study related documents including, study design, writing the abstract of the protocol, concluding the protocols, approval of the study template of informed consent and preparation of the documents.
Conducting a successful study includes, attending the meetings and answering the questions of the management teams, data monitoring committee and other teams.
Data reviewing responsibilities includes, reviewing the patient data along with vital
signs and abnormalities, approving patient narratives and GCP guidelines.
Making the study team capable of resolving the issues, deciding inclusion and
exclusion criteria, studying closeout and inspection readiness.
How to become a certified medical monitor:
MD, MBBS, FMG, IMG are eligible to apply for the certification and for these non clinical physician jobs (learn more at nonclinicaldoctors.com). These health care professionals will be able to get adequate knowledge and experience to deal with the clinical trials. These professionals would have the ability to deal with the adverse events occurring during the trials. After medical monitor training and certifications; chances of their hiring by CRO’s (contract research organization) would increase. Unmatched MDs can pursue this as a phenomenal career path (see unmatchedMD.com).
CCRPS is here to provide one of the most-advanced courses related to medical monitor: https://app.ccrps.org/courses/medial-monitor-certification
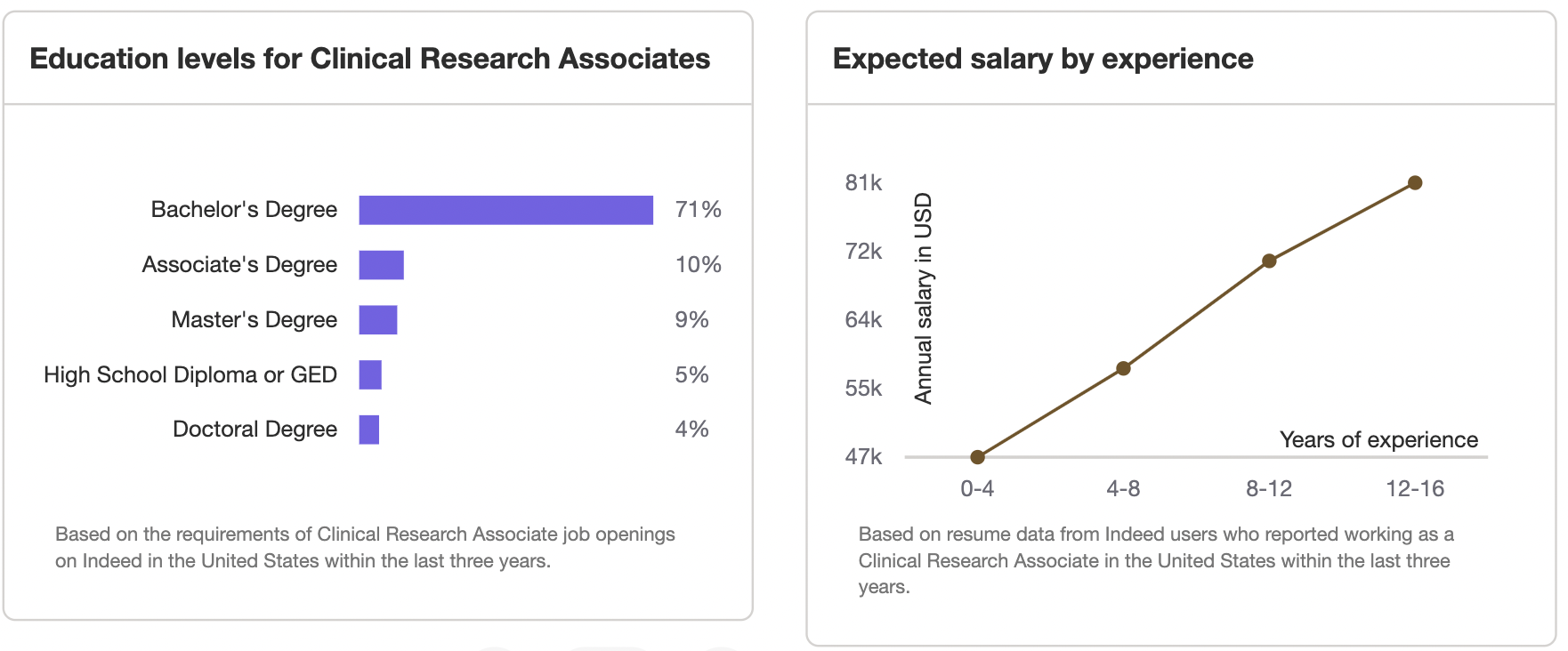
Clinical Research Associate Salary - What's the pay for a clinic research associate?
Here's What You Need to Know to Get a Clinical Research Associate Job
What's the pay for a Clinical Research Associate?: $61-$110K
What's the pay for a Clinical Research Associate?: $61-$110K
A Clinical Research Associate (or Monitor) is hired either in-house (“the trial site”) or externally (by the sponsor or CRO) to review clinical trial data and ensure that investigational therapies are tested ethically and scientifically through performing site visits that review files like patient medical notes in order to ensure quality of trial data. The catch 22 of clinical research associate jobs is that the ICH GCP guidelines require both education AND experience in order to work in this role, so getting your foot in the door is tough. Once you get experience, your education (i.e., certifications or degrees showing understanding of additional responsibilities) can help promotes you quickly through the CRA career ladder. For those looking to understand more about these guidelines, the ICH-GCP course might be a crucial step.
How to become or get promoted as a clinical research associate?
Having a certification through CCRPS’s accredited Advanced Clinical Research Associate Certification (CRA) course can help professionals 1) get promoted 2) get a raise 3) improve efficiency 4) get hired as a Clinical Research Associate. Additionally, those interested in broader roles in clinical research may consider the Clinical Research Coordinator course, the Pharmacovigilance Certification, or the Clinical Trials Assistant Training.
How much does a Clinical Research Associate make in the United States?
The average Clinical Research Associate salary in the United States is $61-110K. Salary ranges can vary widely depending on many important factors, including education, certifications, additional skills, and the number of years you have spent in your profession. For those looking at a leadership role, the Advanced Clinical Research Project Manager Certification or the Advanced Principal Investigator Physician Certification could be relevant. Furthermore, the Medical Monitor Certification can enhance skills for those specifically interested in medical monitoring aspects.
Clinical Research Associate (CRA) Salary
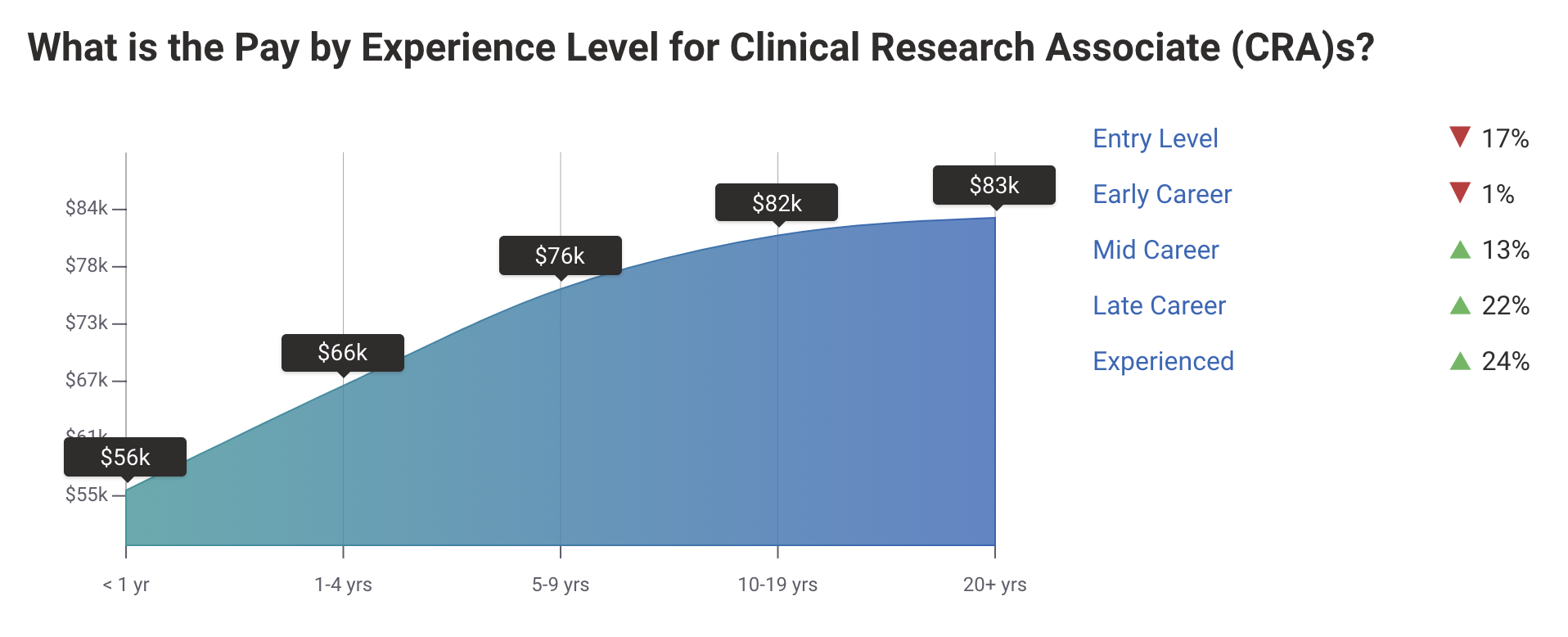
Per Payscale “An entry-level Clinical Research Associate (CRA) with less than 1 year experience can expect to earn an average total compensation (includes tips, bonus, and overtime pay) of $55,588 based on 203 salaries. An early career Clinical Research Associate (CRA) with 1-4 years of experience earns an average total compensation of $66,245 based on 1,118 salaries. A mid-career Clinical Research Associate (CRA) with 5-9 years of experience earns an average total compensation of $76,086 based on 294 salaries. An experienced Clinical Research Associate (CRA) with 10-19 years of experience earns an average total compensation of $81,540 based on 157 salaries. In their late career (20 years and higher), employees earn an average total compensation of $83,342.”
Determine CRA Salary by location using Payscale
Salary: Clinical Research Associate
Research Associate Salary resource: Click here to see the CRA Salary Range from 1,800+ employers
Clinical Research Associates Pay: Clinical Research Associate Salary in United States (by city)
Durham, NC - $97K
New York, NY, Irvine, CA, Houston, TX - $95K
Philadelphia, PA - $92K
Atlanta, GA - $84K
Raleigh, NC - $82K
Chicago, IL - $79K
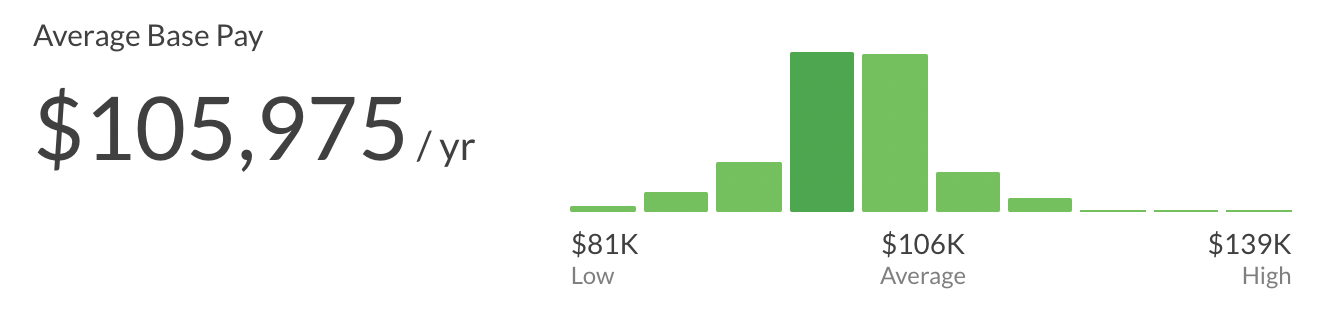
Senior Clinical Research Associate Salary
The average salary of a Senior Senior Clinical Research Associate is ~105K per year ($54/hour, $2k/week, $9k/month). This can range from $81k to $139k.
Starting Salary of a Clinical Research Associate Position is between $60,000 and $65,000
Some employers may prefer hiring entry-level clinical research associates with less experience in clinical research so they can learn their job functions by training under Senior CRAs or CRA IIs.
Clinical Research Associate II Salary is $86,677 / yr
CRA II @ PPD: $84,733/yr
CRA II @ PRA Health Sciences: $96,017/yr
CRA II @ IQVIA: $79,412/yr
CRA II @ ICON: $100,000/yr
Comment below how much you get paid and what helped you get promoted!
Having a certification through CCRPS’s accredited Advanced Clinical Research Associate Certification course can help professionals 1) get promoted 2) get a raise 3) improve efficiency 4) get hired as a Clinical Research Associate.