Medical Monitoring in Clinical Research - Non Clinical Physician Jobs
What Is medical monitor in Clinical Research?
A Full Overview and Guide to Becoming a Physician Medical Monitor
Considered a “Hidden Gem” for Non Clinical Physician Jobs
Clinical trials are the bedrock of medical progress, meticulously evaluating the efficacy and safety of novel drugs and treatments. Within this vital process, medical monitors (MMs) play an indispensable role. These physician-experts oversee critical aspects of the trial, guaranteeing both patient well-being and the scientific integrity of the research. For those interested in this role, consider our Medical Monitor Certification.
Distinct Expertise: Physician-Level Oversight Makes the Difference
While clinical research associates (CRAs) ensure protocol adherence and accurate adverse event (AE) reporting at trial sites, medical monitors bring a physician's perspective. They leverage their medical knowledge to:
Guide Protocol Development: Medical monitors advise on designing protocols that prioritize patient safety and align with current best practices as outlined by the National Institutes of Health (NIH) in their guidance document, "Clinical Trial Design Considerations for Safety" NIH Guidance. For those looking to further understand protocol development, consider our ICH-GCP course.
Evaluate Safety Concerns: Throughout the trial, they assess and address patient safety issues that may arise, referencing the Food and Drug Administration's (FDA) "Guidance for Investigators for Conduct of Human Clinical Trials" FDA Guidance for best practices.
Make Unblinding Decisions: In rare cases of severe AEs, medical monitors determine if unblinding is necessary for effective intervention, following the European Medicines Agency's (EMA) ICH Guideline E6(R2) on Good Clinical Practice EMA Guidance. Aspiring professionals can deepen their understanding with our Advanced Clinical Research Project Manager Certification.
Beyond Oversight: Fostering Effective Collaboration
Similar to CRAs, medical monitors act as liaisons between sponsors and trial sites. They review and ensure proper coding and reporting of AEs, guaranteeing data accuracy and adherence to regulatory guidelines set forth by organizations like the International Council for Harmonisation of Good Clinical Practice (ICH-GCP). For further training in these responsibilities, explore our Clinical Research Coordinator course and Pharmacovigilance Certification.
The Multifaceted Role of the Medical Monitor
The complexities of clinical research in 2024 demand a multifaceted role for medical monitors. Their responsibilities encompass:
Protocol Review and Design: Scrutinizing protocols to prioritize patient safety and ensure alignment with best practices. Consider our CRA course for detailed insights into this role.
Safety Oversight: Continuously monitoring patient well-being throughout the trial and addressing any safety concerns.
Data Analysis and Interpretation: Collaborating with the research team to analyze and interpret trial data effectively.
Regulatory Compliance: Ensuring the trial adheres to all relevant regulatory requirements.
Communication and Collaboration: Acting as a bridge between sponsors, investigators, and research teams, fostering clear communication and collaboration.
Specialist Services for Streamlined Trials
Contract research organizations (CROs) and sponsors often partner with specialist companies like C3i Solutions and GeorgeClinical to leverage their expertise in providing medical monitor services. For those aspiring to excel in assisting clinical trials, our Clinical Trials Assistant Training offers comprehensive insights.
Additionally, for those interested in reaching the pinnacle of research oversight, consider the Advanced Principal Investigator Physician Certification.
Essential Resources
National Institutes of Health (NIH): Role of Medical Monitors in Safety Oversight https://www.niaid.nih.gov/sites/default/files/medicalmonitor.pdf
Explore Our Clinical Research Training Programs:
Key Distinctions: Medical Monitor vs. Other Clinical Trial Roles
It's crucial to distinguish the medical monitor from other vital clinical trial personnel. Here's a quick breakdown:
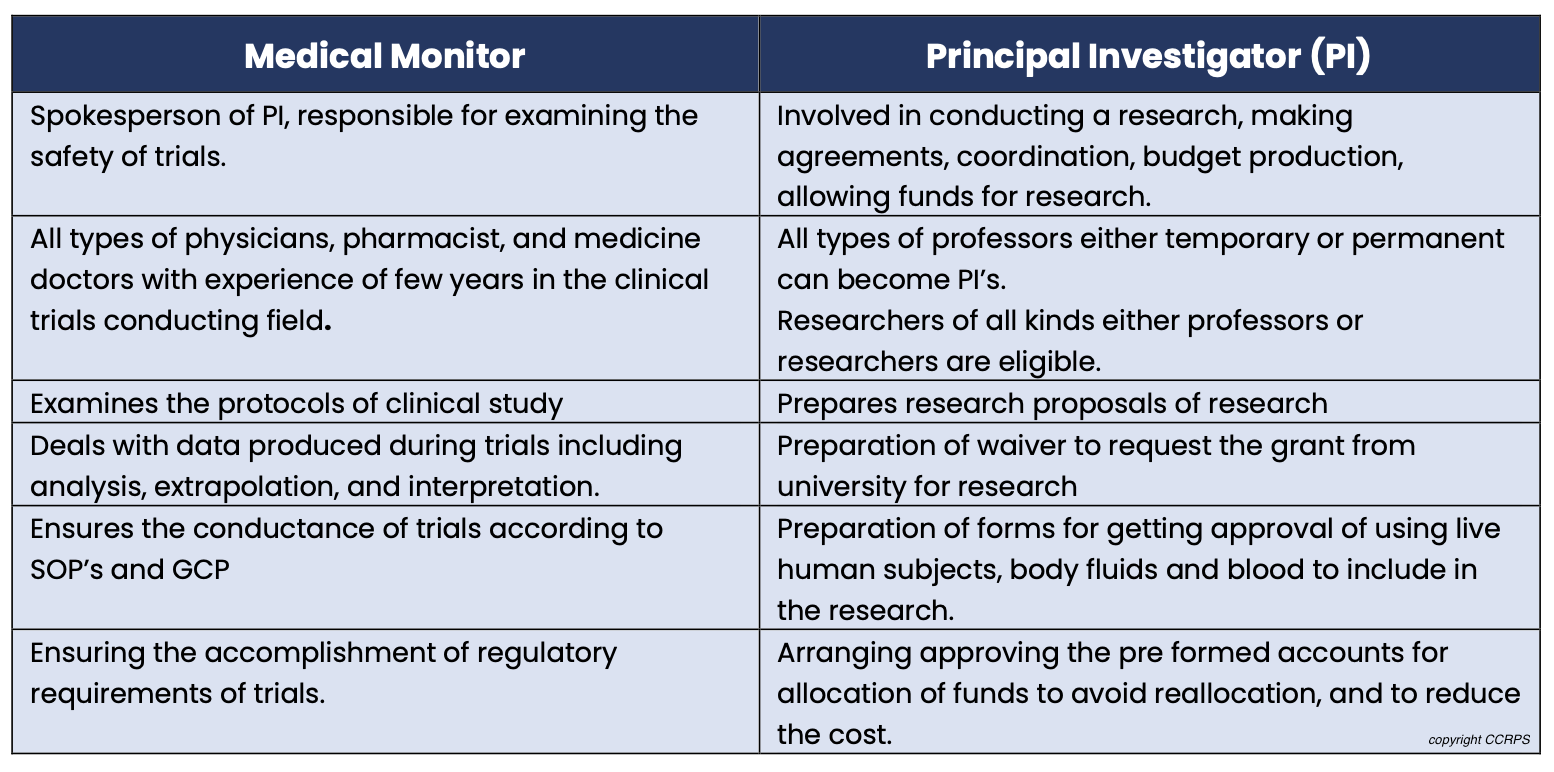
Medical Monitor vs. Principal Investigator (PI): PIs lead trials at a specific site, while medical monitors offer broader oversight across multiple trials and sites.
Medical Monitor vs. Clinical Research Associate (CRA): CRAs focus on protocol adherence and data collection at a single site, whereas medical monitors provide physician-level expertise for broader trial aspects.
Understanding Medical Monitoring According to E6-GCP
The E6 Good Clinical Practice (GCP) guidelines define a medical monitor as a person responsible for supervising the clinical trial process. They ensure that protocols, standard operating procedures (SOPs), Good Manufacturing Practices (GMP), and regulatory requirements are all followed according to established standards as outlined in the European Medicines Agency's (EMA) ICH Guideline E6(R2) on Good Clinical Practice https://www.ema.europa.eu/en/human-regulatory-overview/research-and-development/compliance-research-and-development/good-clinical-practice
Alternative Terminologies for Medical Monitor
It's important to note that medical monitor is not the only term used for this role. Here are some alternatives:
Clinical research associate (CRA)
Site manager
Senior CRA
Clinical trial assistant (CTA)
However, clinical research associate (CRA) is the most frequently used alternative. Be aware that while there may be some overlap in responsibilities, CRAs
Difference between a Medical Monitor and Principal Investigator
What is a medical monitor in clinical research
Salary of a medical monitor
Salary of medical monitor varies due to different factors including education, certifications, additional skills, number of experience years in profession.
The average salary of a medical monitor is 155,000$ and salary usually ranges from 87,000$- 398,000 (Ziprecruiter).
The national average for CRA is $65-119k which is different than medical monitors working in the pharmaceutical companies.
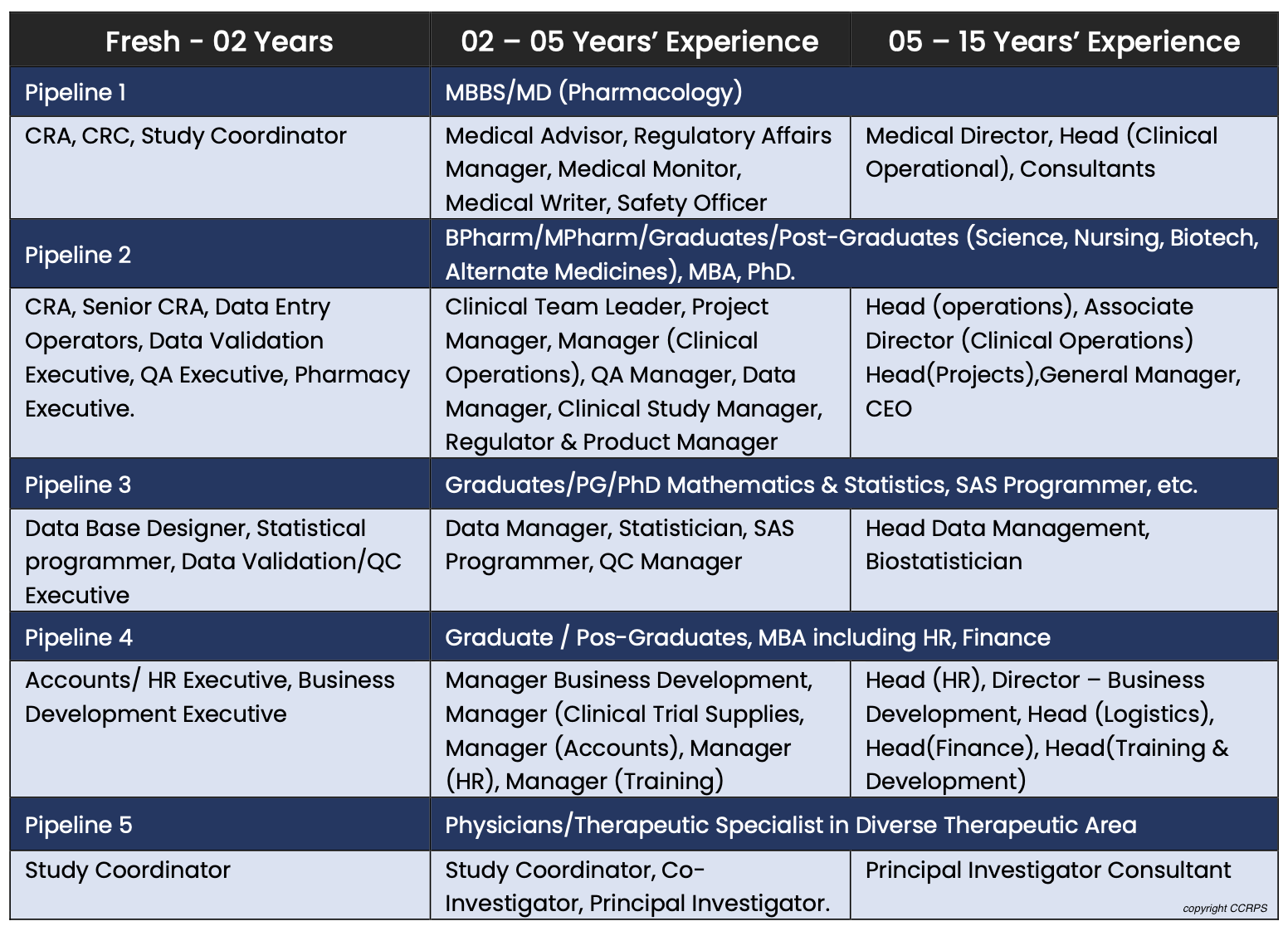
There are so many opportunities in the clinical research industry. At the site level, some are study coordinator, principal investigator, sub investigator, research assistant. At the CRO level there are CRA's, CTA's, CMA's, medical monitors, project managers. At a Sponsor level there are dozens more. What is good about many of these, is one's ability to "level up" over time into more desirable and higher paying positions.
Skills and qualifications required to be a medical monitor:
Medical degree with strong leadership skills
Direct experience in the pharmaceutical industry (i.e. Clinical Research Associate; preferred; although with CCRPS Medical Monitor Certification you are qualified to work as both a CRA and MM)
The preferred countries for the experience are US and EU
Knowledge of both local and Global regulatory requirements as well as the
knowledge of local and Global GCP
Skillful in converting input into regulatory documentation.
Strong communication skills to deal with stakeholders either internal or external.
Strong writing and presentation skills.
Skillful to deal with audience especially medical and scientific community.
Relevant work experience in medical monitoring and/or pharmacovigilance
and/or drug safety experience in a CRO, pharmaceutical, or clinical trial environment required.
Must have appropriate understanding about the International conference on Harmonization (ICH), GCP guidelines.
Must have appropriate knowledge of research, clinical trials and clinical terminologies.
Having strong decision-making power to deal with the trials.
Proficiency in English (written and verbal) required.
Capable of building and maintaining the trust of clients.
You can showcase and gain the knowledge you need to work as a MM with CCRPS’s Medical Monitor certification which trains you to a Senior Monitor’s level of knowledge to allow for easy promotion in the field. You do need experience before getting promoted so we suggest getting an internship while studying for the course prior to applying for Medical Monitor or CRA jobs!
Individuals who should try to be a medical monitor include:
Typically this is a fully qualified physician (MD, MBBS, IMG, FMG)
Sometimes residency in the work-specific department or in internal medicine is helpful (whether international or US-completed)
In the US it is also permitted to also use a PharmD (pharmacist) as a medical monitor
Role and responsibilities of a Medical monitor:
At the clinical trials site, role of the monitor depends upon the experience of the monitor as well as the phase of the ongoing trial. A monitor is a competent person performing differant responsibilities and activities at different sites in accordance with the phases of trials. Some of these responsibilities are:
While discussing and presenting the facts and initiating a trial with investigator a monitor must become a mentor and counsellor.
Responsible for developing devices and conducting research on drugs in improving the quality of life of patients.
Providing monitoring reports, the guidance about further actions, lacking in the study, elaborating findings to improve compliance.
Dealing with the recruitment of the research patients and their retention or exclusion for obtaining quality findings.
Situation handling is the main responsibility of the monitor to safely resolve the issue by troubleshooting the problem.
Planning of travelling costs and schedules are also maintained by the monitor.
A monitor works as a detector when it comes to decide a site for trial and visiting the
sites.
Effective communication and convincing ability while dealing with different levels of
the people is the major skill and responsibility of the medical monitor.
Developing regulatory and study related documents including, study design, writing the abstract of the protocol, concluding the protocols, approval of the study template of informed consent and preparation of the documents.
Conducting a successful study includes, attending the meetings and answering the questions of the management teams, data monitoring committee and other teams.
Data reviewing responsibilities includes, reviewing the patient data along with vital
signs and abnormalities, approving patient narratives and GCP guidelines.
Making the study team capable of resolving the issues, deciding inclusion and
exclusion criteria, studying closeout and inspection readiness.
How to become a certified medical monitor:
MD, MBBS, FMG, IMG are eligible to apply for the certification and for these non clinical physician jobs (learn more at nonclinicaldoctors.com). These health care professionals will be able to get adequate knowledge and experience to deal with the clinical trials. These professionals would have the ability to deal with the adverse events occurring during the trials. After medical monitor training and certifications; chances of their hiring by CRO’s (contract research organization) would increase. Unmatched MDs can pursue this as a phenomenal career path (see unmatchedMD.com).
CCRPS is here to provide one of the most-advanced courses related to medical monitor: https://app.ccrps.org/courses/medial-monitor-certification
Clinical Research Careers in India
Clinical Research Courses in India - US Certification
Your Fast Track to a Rewarding Career as a Clinical Research Associate (CRA) in India
The world of clinical research is booming, and India is a hotspot for exciting opportunities. If you're looking for a career that combines science, travel, and the chance to make a real difference in people's lives, then becoming a Clinical Research Associate (CRA) might be the perfect fit!
Why be a CRA in India?
Financial Security: The average salary for a CRA in India is ₹342,978, with experienced CRAs earning significantly more (Salary reference: Glassdoor https://www.glassdoor.co.in/Salaries/clinical-research-associate-salary-SRCH_KO0,27.htm). Entry-level CRAs can expect to start around ₹298,118, and experienced CRAs can reach an average of ₹696,343.
Travel Perks: CRAs often travel to different research sites, offering a chance to experience new places and cultures.
Global Impact: Your work contributes to the development of new medications and treatments, impacting lives worldwide.
Becoming a CRA in India: A Step-by-Step Guide
Education: A Bachelor of Science (B.Sc.) in a science field like biology, chemistry, or pharmacy is the minimum requirement. However, a Master's degree (M.Sc., M.Pharma, or Ph.D.) will make you a more competitive candidate.
Experience: While not always mandatory, 1-2 years of experience in research or healthcare will give you a head start. Consider internships or volunteering opportunities to gain relevant skills.
Certification: Good Clinical Practice (GCP) certification demonstrates your understanding of ethical research practices and is highly valued by employers.
Additional Tips:
Network: Attend industry events and connect with professionals on LinkedIn to learn more about the field and find potential opportunities.
Highlight Your Skills: Develop strong communication, organizational, and problem-solving skills. Attention to detail is crucial for CRAs.
Ready to Take the Next Step?
Explore our website for resources on becoming a CRA, including articles on the international job market. We can also help you find India-specific CRA training programs at competitive prices.
Clinical Research Coordinator: Interested in managing and overseeing clinical trials? Consider becoming a Clinical Research Coordinator: https://app.ccrps.org/courses/Clinical-Research-Coordinator.
Pharmacovigilance Certification: Specialize in monitoring the safety of pharmaceuticals by exploring the Pharmacovigilance Certification: https://app.ccrps.org/courses/pharmacovigilance-certification.
Clinical Research Associate (CRA): Learn to monitor clinical trials effectively and ensure compliance with regulations by becoming a Clinical Research Associate (CRA): https://app.ccrps.org/courses/cra.
ICH-GCP (International Conference on Harmonisation - Good Clinical Practice): Gain an understanding of the ethical and quality standards in clinical research through the ICH-GCP course: https://app.ccrps.org/courses/ich-gcp.
Getting Started in Clinical Trials
Clinical Trials Assistant Training: Starting your career in clinical trials? Consider the Clinical Trials Assistant Training: https://app.ccrps.org/courses/Clinical-Trials-Assistant-Training to get foundational skills.
Advanced Clinical Research Careers
Advanced Clinical Research Project Manager Certification: Aiming to lead clinical research projects? The Advanced Clinical Research Project Manager Certification: https://app.ccrps.org/courses/Advanced-Clinical-Research-Project-Manager-Certification might be the next step.
Advanced Principal Investigator Physician Certification: Aspire to lead clinical trials? Consider obtaining the Advanced Principal Investigator Physician Certification: https://app.ccrps.org/courses/Advanced-Principal-Investigator-Physician-Certification.
Medical Monitor Certification: Specialize in monitoring the medical aspects of clinical trials with the Medical Monitor Certification: https://app.ccrps.org/courses/medial-monitor-certification.
Email us for more information on finding the perfect program to launch your exciting career in Clinical Research!