Why Are So Many Students Taking Clinical Research Courses?
The rise in student enrollment in clinical research courses isn't just a trend—it's a movement! With the healthcare landscape evolving rapidly, the demand for skilled professionals who can navigate the complexities of clinical trials is skyrocketing. But what's drawing students into this intricate field of study? From the thrill of discovering new treatments to the satisfaction of impacting public health policies, the reasons are as compelling as they are vital.
Understanding Clinical Research
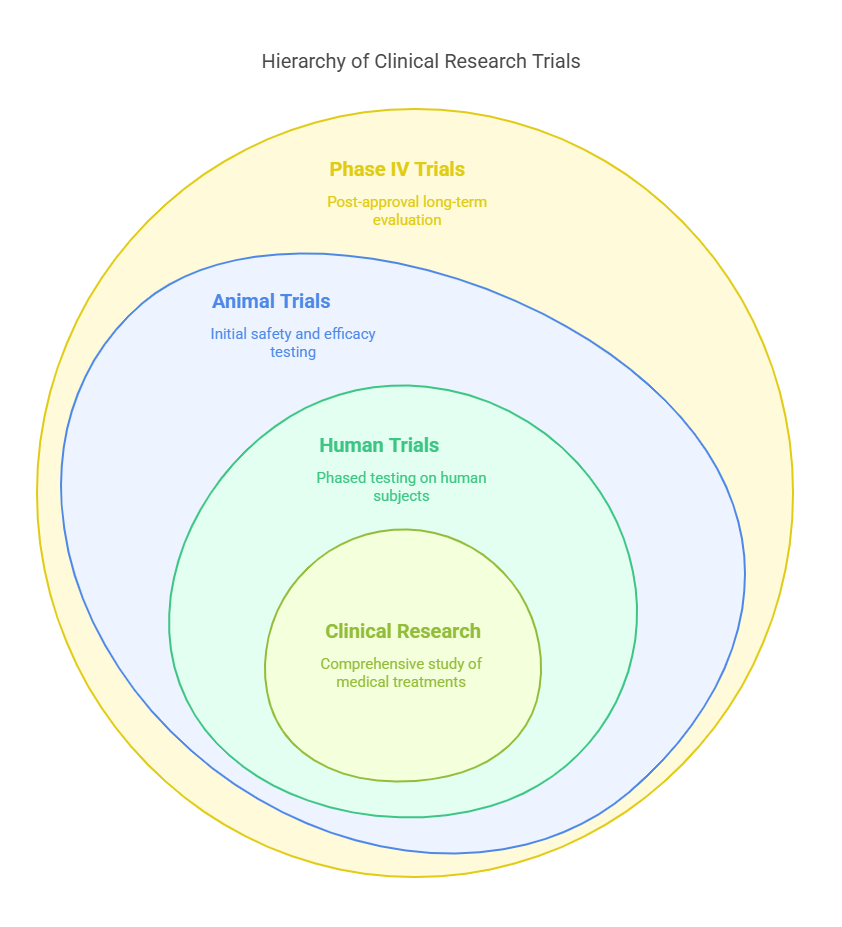
A Brief Overview Clinical research splits into two main arenas: animal and human trials, each critical for validating the safety and efficacy of new medical solutions. These studies are meticulously planned to answer crucial questions about patient eligibility, treatment protocols, and study duration, ensuring that every trial contributes to medical advancements safely and ethically.
Animal Trials
Animal trials, or preclinical studies, involve testing new treatments or drugs on animals to assess their safety and effectiveness before they are used in humans. This step is critical as it helps researchers identify potential side effects and determine the proper dosages that can be safely administered to humans. These studies are typically the first line of investigation in clinical research and are necessary before moving on to human trials.
Human Trials
Once a treatment has shown promise in animal trials, the research moves into human trials, which are conducted in several phases:
Phase I tests the safety of a drug or treatment on a small group of people.
Phase II expands the testing to a larger group to evaluate the efficacy and further assess safety.
Phase III involves a larger population to confirm effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug or treatment to be used safely.
Phase IV occurs after the FDA has approved the medication or treatment. This phase aims to gather more information on the drug's risks, benefits, and optimal use in diverse populations over a longer timeframe.
Key Elements of Clinical Research
Patient Eligibility: Before participating in a trial, participants must meet specific criteria that typically include factors like age, gender, the stage of a disease, previous treatment history, and other medical conditions. These criteria are designed to ensure the safety of participants and the validity of the data collected.
Treatment Protocols: These are the plans that outline how the clinical trial will be conducted. Protocols define what treatments participants receive, the dosage and frequency of the treatment, what assessments they will undergo during the trial, and the length of the study. Ensuring that protocols are meticulously planned and followed is essential for the credibility and success of the trial.
Study Duration: The length of the study is crucial as it affects the quality and reliability of the clinical data collected. Longer duration can provide more data on long-term efficacy and side effects, while shorter studies might focus on preliminary assessments of safety and effectiveness.
Ethical Considerations
Ethical considerations are paramount in clinical research to protect the rights and well-being of participants. This involves informed consent, where participants are fully informed about the study’s risks and benefits and their right to withdraw from the study at any time without any consequences to their usual care.
Clinical research must adhere to ethical standards and regulatory requirements to ensure that the data collected are legitimate and that the rights and health of all participants are protected. By meticulously planning and ethically conducting these studies, clinical research contributes significantly to medical advancements, offering new solutions that can enhance patient care and treatment options globally.
The Lure of Clinical Research Courses
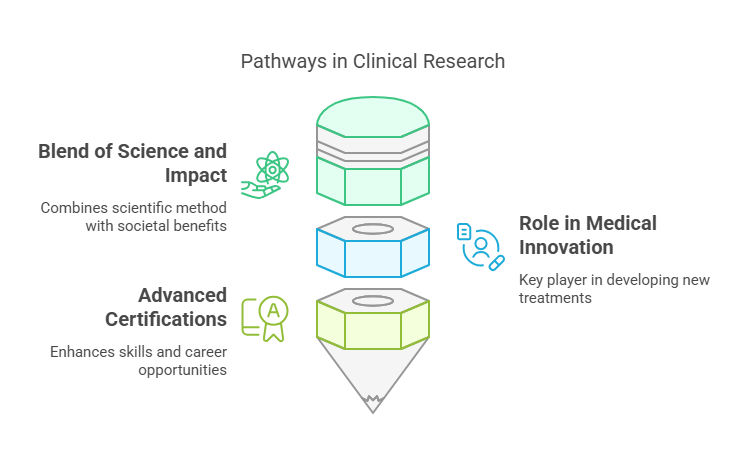
Why choose a career in clinical research? For many, it's the blend of science and impact. Clinical research professionals are at the forefront of medical innovation, turning theoretical health solutions into life-saving treatments. Advanced certifications, like the Clinical Research Project Manager or Principal Investigator Certifications, only broaden these opportunities, enhancing both skill sets and career trajectories.
Blend of Science and Impact
Clinical research offers a unique blend of rigorous scientific method and the potential for significant societal impact. Professionals in this field use scientific principles to design and implement studies that test the effectiveness and safety of new medical treatments, drugs, and devices. The ultimate goal is not just to advance scientific knowledge but to translate these discoveries into real-world applications that can improve patient outcomes and even save lives.
Role in Medical Innovation
Clinical research professionals are integral to the medical innovation pipeline. They are involved at nearly every stage of the development process of new medical treatments—from the initial concept and preclinical testing to clinical trials and the eventual market release of new therapies. Their work ensures that any new treatment is both safe for patients and effective, based on rigorous, data-driven evaluations.
Turning Theoretical Health Solutions into Life-Saving Treatments
One of the most compelling aspects of clinical research is the ability to turn theoretical health solutions into practical, life-saving treatments. This involves not only testing new drugs or therapies to observe their effectiveness but also meticulously monitoring side effects and adjusting dosages to optimize safety and efficacy. The process is complex and requires a deep understanding of biology, pharmacology, and medicine, as well as a commitment to patient safety and ethical standards.
Advanced Certifications
Advancing in a clinical research career often involves gaining specialized knowledge and skills through advanced certifications. These certifications, such as the Clinical Research Project Manager or Principal Investigator Certifications, are crucial for those aiming to take on leadership roles within the field. They provide:
Specialized Knowledge: Deep dives into specific areas of clinical research, such as project management, trial design, regulatory compliance, and ethical considerations.
Enhanced Skill Sets: Development of advanced skills including team leadership, data analysis, and critical problem-solving, which are essential for managing complex research projects and navigating the challenges of clinical trials.
Career Trajectory Enhancement: With advanced certifications, professionals can access higher-level positions and opportunities within academia, the pharmaceutical industry, and governmental agencies. These roles not only offer better compensation but also greater influence over the direction and outcomes of clinical research projects.
Why People Choose This Career
People are drawn to careers in clinical research for several reasons:
Intellectual Stimulation: The field is constantly evolving, presenting new challenges and requiring continual learning and adaptation.
Impact on Public Health: Clinical researchers play a direct role in improving health outcomes and advancing public health by developing new treatments and therapies.
Personal Fulfillment: Many find personal satisfaction in knowing that their work contributes directly to saving lives and improving the quality of life for patients around the world.
Path in Clinical Research
Navigating the world of clinical research education can seem daunting. With options ranging from undergraduate degrees to doctoral programs, and specializations like ICH-GCP or Medical Monitor Certifications, the journey is as diverse as the field itself. Prospective students should focus on institutions renowned for their robust medical programs and hands-on training opportunities.
Range of Educational Options
Clinical research education spans multiple levels, each catering to different career stages and professional goals:
Undergraduate Degrees: These are typically bachelor’s degrees in fields like biology, pharmacology, nursing, or public health. These programs provide foundational knowledge in life sciences and introduce students to the basics of clinical research, including study design, statistical analysis, and ethical considerations.
Master’s Degrees: For those who want to delve deeper or specialize in a particular aspect of clinical research, master’s programs offer advanced training. These degrees often focus on more specific areas like clinical trial management, regulatory affairs, or data management. They are suitable for students coming from a life sciences background or professionals aiming to advance their careers.
Doctoral Programs: PhD programs or doctorates in clinical research are geared towards those interested in leading research projects or entering academia. These programs involve extensive research work, culminating in a dissertation that contributes new knowledge to the field.
Specializations and Certifications
Beyond degree programs, there are specialized certifications that enhance a clinical researcher’s credentials and expertise:
ICH-GCP (International Council for Harmonisation - Good Clinical Practice): This is a standard for the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials. Certification in ICH-GCP ensures that a professional is knowledgeable about the global standard protecting the rights, safety, and well-being of trial subjects and ensuring that clinical trial data are credible.
Medical Monitor Certifications: These are specialized credentials for those who wish to oversee the medical aspects of clinical trials. Medical monitors are responsible for the safety of trial participants and are involved in making critical decisions related to the continuation of the trial, modifying protocols, and ensuring compliance with ethical standards.
Choosing the Right Institution
When selecting an institution for clinical research education, prospective students should consider several factors:
Reputation and Accreditation: Institutions renowned for their robust medical or pharmaceutical sciences programs are preferable, as they are likely to provide high-quality education and have established ties with healthcare industries and research organizations.
Hands-On Training Opportunities: Practical experience is crucial in clinical research. Institutions that offer internships, lab work, and opportunities to participate in actual clinical trials give students a competitive edge by providing real-world experience alongside academic learning.
Faculty Expertise: Learning from experienced professionals who are actively involved in clinical research can enhance the educational experience, offering insights into the latest challenges and innovations in the field.
Conclusion
Clinical research is a gateway to innovation, patient safety, and global healthcare advancements. With the right education, certifications, and hands-on training, you can build a rewarding career in this dynamic field. CCRPS provides industry-leading programs to help you gain the expertise needed to excel. Take the next step and shape the future of medicine today!
Explore Courses for Clinical Research Career
Courses Available: