The Basics of Clinical Trial Data Management
Clinical trial data management (CTDM) is a crucial aspect of any clinical research endeavor. It involves the process of collecting, organizing, validating, analyzing, and reporting data throughout the life cycle of a clinical trial. In an industry that is highly regulated, ensuring data accuracy, integrity, and compliance with international standards is paramount. Whether it’s for regulatory approval from agencies such as the FDA or EMA, or for internal use within pharmaceutical companies, clinical trial data management ensures the reliability and transparency of the data supporting the trial outcomes.
This blog will explore the foundational principles of data management in clinical trials, covering everything from data lifecycle management to key data integrity practices and the tools employed by clinical data managers.
What is Data Management in Clinical Trials?
Clinical trial data management refers to the collection, organization, validation, and analysis of data obtained during a clinical trial. It is a multi-step process that ensures the data is accurate, complete, and consistent, enabling researchers to make informed decisions about the safety and efficacy of the treatment being tested.
In clinical trials, data can be obtained from a variety of sources, including patient records, laboratory results, and questionnaires. This raw data must be processed and organized into a structured format that is suitable for analysis and reporting. Data management professionals ensure that this data is cleaned, checked for errors, and prepared in such a way that regulatory bodies can trust the results.
The role of data management in clinical trials is pivotal for the following reasons:
Regulatory Compliance: Ensuring the trial adheres to the standards set by regulatory agencies like the FDA or EMA.
Data Integrity: Maintaining accuracy and completeness of the data to ensure its validity.
Timely Decision Making: Providing the necessary information for researchers and sponsors to make informed decisions about trial progress.
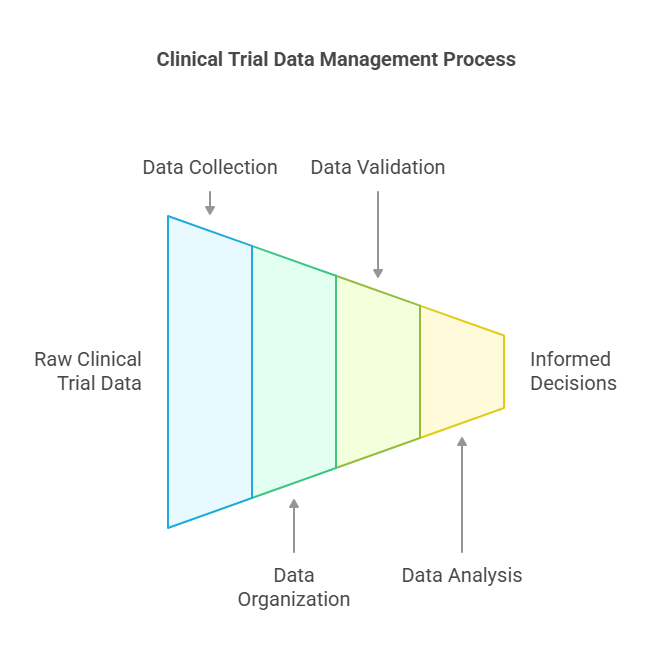
The Data Lifecycle in Clinical Trials
The data lifecycle in clinical trials can be broken down into four essential phases: collection, validation, analysis, and reporting. Each phase has its own set of tasks that require careful management to ensure that the data supports reliable and reproducible results.
Data Collection: Data collection begins as soon as the clinical trial starts and continues throughout the trial. Data can come from various sources:
Electronic Health Records (EHR): Data from patient medical histories, lab tests, and other clinical observations.
Patient-Reported Outcomes (PRO): Self-reported data from patients regarding their symptoms, side effects, or quality of life.
Clinical Assessments: Data collected by clinical staff during patient visits, including physical examinations, tests, and measurements.
Modern clinical trials often use electronic data capture (EDC) systems to gather and store data directly from clinical sites, which minimizes errors from manual data entry.
Data Validation: Data validation involves ensuring the collected data is both accurate and consistent. This stage includes:
Data Cleaning: Identifying and correcting errors in the data, such as missing values, out-of-range values, or duplicate records.
Consistency Checks: Ensuring that the data follows the logical rules defined by the clinical protocol. For instance, a patient’s reported age should align with their date of birth.
Source Data Verification: Confirming that the data entered into the system corresponds to the original source (e.g., patient records or lab reports).
This phase is critical for ensuring data integrity, which is fundamental for maintaining the trial's credibility and trustworthiness.
Data Analysis: Data analysis is the phase where the cleaned data is processed to extract meaningful insights. Statistical methods are applied to determine the effectiveness and safety of the treatment. Common analysis types include:
Descriptive Statistics: Summarizing the characteristics of the data, such as mean, median, and standard deviation.
Inferential Statistics: Drawing conclusions about the broader population based on the sample data.
This stage also includes determining any correlations, trends, or anomalies within the data that could influence the trial's outcomes. The data needs to be processed with precision to ensure it provides reliable results.
Data Reporting: The final phase involves presenting the analyzed data in a report format suitable for submission to regulatory agencies, publication, or dissemination to stakeholders. This report includes:
Summary of Findings: The trial’s primary results, conclusions, and any recommendations for further research.
Compliance Documentation: Evidence that the trial adhered to regulatory requirements and standards (e.g., FDA Good Clinical Practice).
Data Visualizations: Graphs, tables, and charts that help illustrate key findings in a more understandable format.
The final report will undergo rigorous review by stakeholders, including clinical research organizations (CROs), sponsors, and regulatory bodies, to ensure it meets the necessary standards for approval.
Importance of Data Integrity in Clinical Trials
Data integrity is the cornerstone of any clinical trial. It refers to the accuracy, consistency, and reliability of the data collected throughout the trial. Without proper data integrity, trial results could be compromised, leading to incorrect conclusions about the safety or efficacy of a treatment.
Ensuring data integrity involves several practices:
Accurate Data Entry: Data should be entered correctly, without mistakes or omissions.
Consistency Across Sources: Data should be consistent, with no contradictions between various sources (e.g., patient records, laboratory reports).
Audit Trails: Keeping a record of all changes made to the data, ensuring that any modifications can be traced and verified.
Regulatory agencies like the FDA and EMA have strict guidelines for maintaining data integrity. For instance, they require that data must be complete, accurate, and traceable, with proper documentation supporting the results. In addition to regulatory compliance, maintaining high data integrity helps to build trust in the results, ensuring that the clinical trial outcomes are both scientifically sound and ethically responsible.
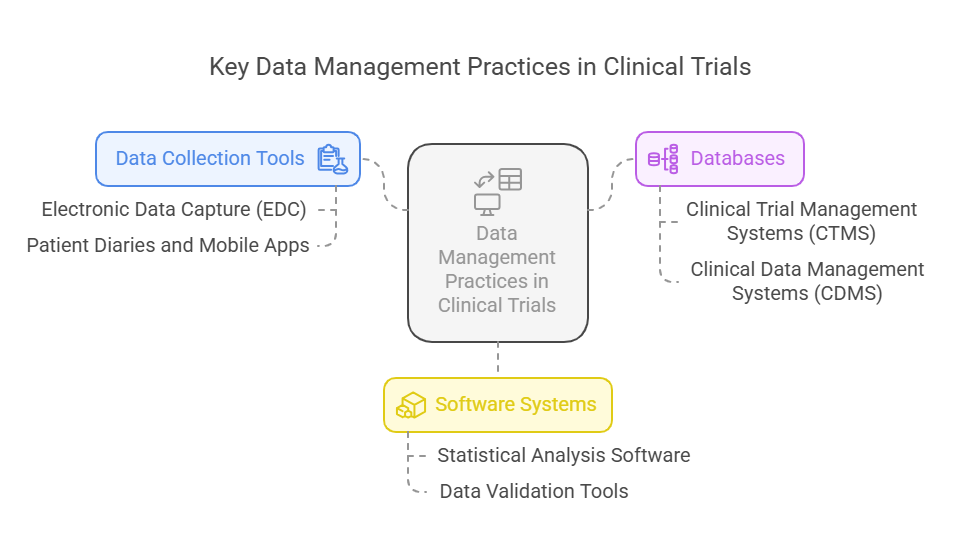
Key Data Management Practices in Clinical Trials
Effective data management relies heavily on the use of the right tools and technologies. Some of the most important practices in clinical trial data management include the use of data collection tools, databases, and software systems that streamline processes and ensure accurate data handling.
Data Collection Tools:
Electronic Data Capture (EDC): Systems like Medidata, Oracle’s InForm, and Veeva Vault are widely used to collect and store data electronically, reducing the risk of manual errors.
Patient Diaries and Mobile Apps: Many trials now use mobile apps for real-time data entry, such as symptom trackers or medication adherence reports. This also helps reduce patient burden and increases data accuracy.
Databases:
Clinical Trial Management Systems (CTMS): These systems, such as Oracle Siebel CTMS, manage trial workflows, track progress, and ensure all data is collected in compliance with the trial protocol.
Clinical Data Management Systems (CDMS): These databases help organize and manage clinical trial data, making it accessible for analysis and reporting.
Software Systems:
Statistical Analysis Software: Tools like SAS, R, and SPSS are commonly used to analyze clinical trial data. These programs offer advanced statistical methods for data analysis.
Data Validation Tools: Tools like OpenClinica and ClinOne are used for validating data during the trial process, ensuring all data points are correctly captured and consistent.
10 Lesser-Known Facts About Clinical Trial Data Management
Electronic Data Capture (EDC) was first introduced in the 1990s, significantly reducing data errors caused by manual entry. (Source)
Data validation checks often use automated scripts to identify errors, reducing the need for human intervention. (Source)
Audit trails in clinical trial data management systems allow for complete transparency of any changes made to trial data. (Source)
Real-time data access has become common in modern clinical trials, allowing stakeholders to monitor data from anywhere. (Source)
Patient-reported outcomes (PROs) are often collected via digital platforms, reducing the burden on patients and improving data accuracy.
Clinical trials with digital endpoints have increased, integrating technologies like wearables for continuous data collection.
Centralized monitoring of trial data is increasingly common, allowing for quicker identification of trends or anomalies.
Cloud-based platforms are now widely used to store clinical trial data, improving scalability and access.
Artificial intelligence (AI) is being explored to automate data cleaning and validation processes.
Blockchain technology is being experimented with to ensure data integrity and reduce the potential for fraud.
Related Blogs
What is Clinical Trial Management?
How Clinical Trials and Data Management Impact Research Outcomes?
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
In the world of clinical trials, managing data effectively is not just about keeping track of numbers—it’s about ensuring that the data can stand up to scrutiny, meet regulatory requirements, and help improve patient care. By following best practices for data collection, validation, and analysis, clinical research organizations can ensure that their trials run smoothly and that their results are reliable.
As clinical trials evolve in 2025, with increasing digitalization and data volume, the role of Clinical Trial Data Management is more critical than ever. CCRPS is here to provide the expertise needed to manage your trial data efficiently and with integrity, ensuring that you stay compliant with industry standards and achieve the highest quality results.
Frequently Asked Questions (FAQs)
-
Data management in clinical trials involves collecting, validating, analyzing, and reporting data to ensure its accuracy, consistency, and compliance with regulatory standards.
-
Data integrity ensures the accuracy and reliability of trial results, which is crucial for regulatory approval and scientific validity.
-
Key tools include Electronic Data Capture (EDC) systems, Clinical Data Management Systems (CDMS), and statistical software like SAS and SPSS.
-
The data lifecycle includes collection, validation, analysis, and reporting, ensuring data is accurate, consistent, and usable for decision-making.
-
The FDA, EMA, and other international regulatory bodies provide guidelines and requirements for data integrity and management in clinical trials.