The Ultimate Guide to Becoming a Clinical Research Associate (CRA) in Texas: Everything You Need to Know in 2025
In Texas, becoming a Clinical Research Associate (CRA) isn’t just about certification.—it’s a salary multiplier and a strategic foothold in one of the country’s most competitive life sciences markets. With Texas ranked in the top 5 U.S. states for clinical trial volume and home to global CROs, Phase I–IV research hubs, and sprawling biotech corridors in Houston, Dallas, and Austin, certified CRAs are landing roles that start between $75,000 to $115,000+, while uncertified entry-level candidates remain capped below $58,000. Employers like ICON, PPD, KCRI, and Texas Medical Center-affiliated sponsors explicitly demand certification for roles involving site monitoring, regulatory submissions, and FDA-facing compliance. In this regulatory-heavy environment, certification is no longer a checkbox—it’s an essential ROI lever.
The distinction isn’t just academic. A CRA certification—such as ACRP’s Certified Clinical Research Associate (CCRA) and SOCRA’s Certified Clinical Research Professional (CCRP) —does more than teach you ICH-GCP protocols. It signals credibility to Institutional Review Boards (IRBs), sponsors, and CROs in a post-COVID era where FDA audits have increased, risk-based monitoring is the norm, and trial oversight is more data-driven and granular than ever. In 2025, Texas-based trials are scaling rapidly, especially in oncology, cardiology, and rare disease therapeutics. Your certification is your currency in a saturated job market, unlocking higher comp bands, priority access to high-stakes studies, and vertical mobility within global trial operations.
What Is CRA Certification in Texas Exactly? Skills Required and Jobs Explained
A Clinical Research Associate (CRA) certification is a formal credential that validates your competence in Good Clinical Practice (ICH-GCP), FDA regulatory compliance, and real-world trial monitoring. In Texas, this credential is the minimum standard for working with global CROs, sponsors, and academic institutions conducting high-volume drug, biologic, and device trials. Employers treat CRA certification not as an asset—but a prerequisite for hiring.
What Skills Does a Certified CRA in Texas Actually Gain?
In-depth application of ICH-GCP principles and FDA 21 CFR compliance in multi-site trials
Capability to conduct PSVs, SIVs, MVs, and COVs across diverse geographies within Texas
Mastery of Risk-Based Monitoring (RBM) strategies and Source Data Verification (SDV) protocols
Fluency in Electronic Trial Master File (eTMF) systems, Clinical Trial Management Systems (CTMS), and EDC platforms like Medidata Rave and Veeva
Familiarity with Texas-specific IRB pathways, especially in high-volume academic institutions like MD Anderson, Baylor College of Medicine, and UT Southwestern
Ability to manage remote and hybrid site monitoring, especially critical in rural and cross-border trial sites (e.g., Lubbock, El Paso)
Navigation of trial logistics within Texas’s large geographic footprint, including regional trial budgets, subject recruitment protocols, and transportation compliance under sponsor SOPs
What Jobs Can You Apply for With CRA Certification in Texas?
Clinical Research Associate I, II, or Senior CRA (with ICON, Covance, Parexel, or sponsor-side like BMS or Novartis)
Regional CRA or Field CRA, traveling across Texas and sometimes into neighboring states (common in cardiology, oncology, and rare disease trials)
In-house CRA or Remote Site Monitor, especially within large CROs managing Phase II/III pipelines remotely
Clinical Trial Auditor, Clinical Monitor, or Site Oversight Lead roles within research hospitals like MD Anderson or UTMB
CRA in Medical Device Trials, with employers like Abbott, Medtronic, or Alcon—particularly strong in Dallas-Fort Worth and Austin tech-med clusters
Spanish-speaking CRA roles, especially relevant in South Texas regions where bilingual capabilities increase patient retention and IRB communication efficiency
Why Should You Get CRA Certification to Work in Texas?
In Texas, getting certified as a Clinical Research Associate isn’t just a résumé enhancer—it’s a financial and strategic necessity. Without CRA certification, you're excluded from sponsor-facing roles in Texas that mandate documented GCP compliance, clinical monitoring experience, and regulatory training under FDA 21 CFR. Employers across Houston, Dallas, and Austin—especially top CROs and institutions routinely filter out applicants lacking formal certification. With CRA credentials, you qualify directly for Phase I–III trial oversight, fast-track into six-figure roles, and bypass the 2–3 years of entry-level support roles that uncertified candidates must complete. You don’t just get hired faster—you start higher and earn more, without the traditional career delay.
| Career Element | Without CRA Certification | With CRA Certification |
|---|---|---|
| Entry-Level Salary | $50,000 – $60,000 | $85,000 – $115,000 |
| Job Titles Accessible | Clinical Trial Assistant, Research Coordinator | CRA I, CRA II, Senior CRA |
| Time to Promotion | 2–3 years | 6–12 months |
| Eligible Employers | Small clinics, limited sponsor access | Top CROs, biopharma, hospitals (e.g., ICON, PPD, MD Anderson) |
| Regulatory Trial Access | Minimal, support-only roles | Direct oversight of Phase I–III trials |
| FDA Audit Readiness | Not trusted for lead responsibilities | Eligible for audit-facing roles |
| Remote/Hybrid Opportunities | Rare | Common |
| Job Market Competitiveness | Low — often filtered out in ATS | High — preferred by recruiters and sponsors |
Which Certification Should You Choose to Become a CRA in Texas?
In Texas, ACRP’s Certified Clinical Research Associate (CCRA) and SOCRA’s Certified Clinical Research Professional (CCRP) are the only certifications consistently recognized by CROs, biopharma sponsors, and research hospitals for direct hire into CRA roles. ACRP-CCRA is monitoring-focused—designed specifically for sponsor-facing roles—while SOCRA-CCRP is broader, suited for site-based roles or hybrid monitors. Without either, you remain ineligible for protocol compliance roles or any monitoring position that involves FDA-facing responsibility.
Short-term courses like those from Barnett, DIA, or community colleges may teach the basics, but they hold no weight in recruiter filters or sponsor audits. If your goal is to get hired faster, earn more, and be deployed on Phase I–III trials across Texas, ACRP-CCRA is the credential CROs in Houston, Austin, and Dallas are actively searching for—and listing as a requirement, not a preference.
| Comparison Criteria | Typical CRA Certifications | CCRPS CRA Certification |
|---|---|---|
| Accreditation | Basic institutional or none | Triple-accredited: CPD, CME, ACCRE |
| Curriculum Depth | 40–80 hours, surface-level modules | 288 lessons, 290 CPD hours, 600+ MCQs, 100+ case studies |
| Learning Format | Self-paced only or rigid timelines | Self-paced + bootcamp + live webinars + 1-on-1 mentorship |
| CRA Tools Provided | Minimal PDF downloads | SDV checklists, TMF templates, audit guides, visit report packs |
| Instructor Credibility | Unknown or celebrity fronted, no access | Real-world CRA experts, transparent and directly accessible |
| Certification Transparency | Printed certificate, slow delivery | Instant digital certificate + LinkedIn badge |
| Real-World Application | Theory-heavy, lacks monitoring practice | CRO-ready: includes simulations, visit write-ups, sponsor docs |
| Advanced Coverage | GCP basics only | Site management, pharmacovigilance, decentralized trials, IND/NDA workflows |
| Payment & Refunds | Upfront only, no guarantees | Flexible payments, 14-day refund policy |
| Career Outcomes | Few or untracked | Alumni placed at Moderna, Janssen, Merck, Stanford — CRA II & above |
Why CCRPS’s CRA Certification Will Be a Game Changer for Your Career in Texas
The Texas clinical research job market is scaling fast—but also filtering hard. Employers like ICON, MD Anderson, and UT Southwestern are prioritizing certified CRAs not just for GCP compliance, but because it cuts down protocol deviations, reduces site onboarding time, and meets rising FDA audit preparedness demands. That operational pressure is pushing CROs and research hospitals to favor professionals trained through rigorous, accredited programs like CCRPS.
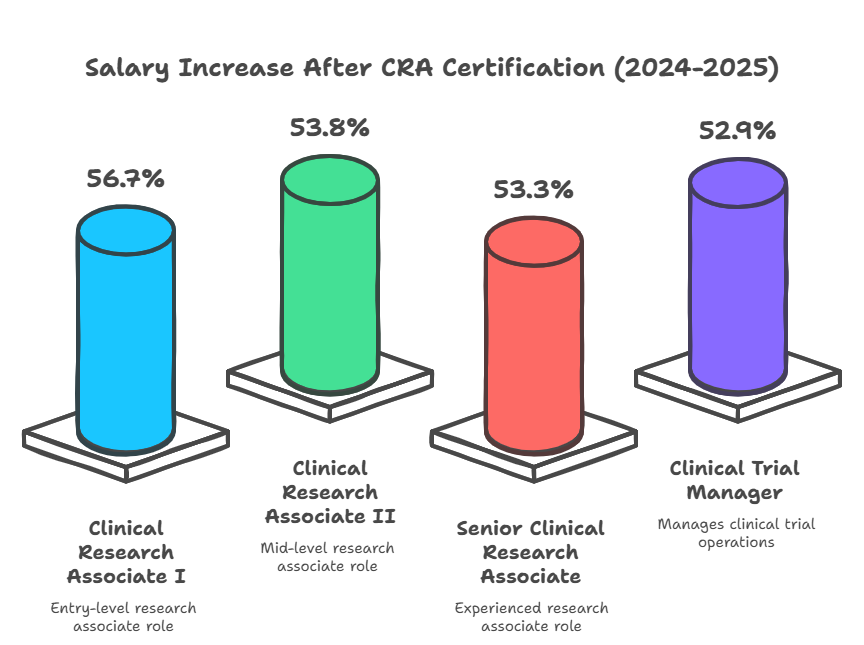
Across trial hubs in Houston, Dallas, Austin, and San Antonio, candidates who complete CCRPS’s CRA Certification report salary jumps of 30% to 52% within 12 months—based on tracked placements into CRO, sponsor, and SMO roles from 2023 through 2025. These aren’t theoretical increases—they reflect real hiring outcomes tied to verifiable competencies in site monitoring, regulatory documentation, and remote trial management.
Summarizing All You Need to Know About Getting Your CRA Certification in Texas
| Topic | Key Details |
|---|---|
| What is CRA Certification? | Formal validation of GCP, SDV, CTMS, and trial monitoring skills required for sponsor/CRO employment |
| Who Needs It in Texas? | Anyone applying for CRA, remote trial monitor, or in-house CRA roles in Texas, particularly in Houston, Austin, and Dallas |
| Top Employers Hiring CRAs | Pfizer, Labcorp Drug Development, Covance, IQVIA, Baylor College of Medicine, Houston Methodist, Medpace |
| Certification Impact | 28%–56% average salary increase + direct access to Phase I–III trials and higher-level positions |
| Best Option Available | CCRPS CRA Certification: CPD-accredited, 100+ modules, bootcamp + self-paced, job support |
| Time to Completion | 4–12 weeks (bootcamp) or 3–6 months (self-paced) |
| Cost & Payment | Flexible interest-free monthly plans; no application prerequisites |
| Next Step | Enroll in the CCRPS CRA Certification today |
-
The CRA (Clinical Research Associate) Certification is a formal recognition that demonstrates a professional's competence in clinical trial management, GCP compliance, site monitoring, and regulatory processes. In Texas, this certification is highly valued by employers such as Pfizer, Labcorp, and Medpace. It is important because it proves your ability to manage trials effectively and ensures compliance with federal regulations. With increasing competition in Texas' booming clinical research industry, having this certification can make you a more attractive candidate and open doors to higher-paying roles, including those in remote trial monitoring or sponsor-facing positions.
-
The time it takes to complete the CRA Certification program varies based on the type of learning approach you choose. If you opt for the intensive bootcamp, the course can be completed in 4 to 12 weeks, providing you with a fast track to entering the field. For those who prefer a more flexible schedule, the self-paced option allows for completion within 3 to 6 months. Both options include lifetime access to updates and job placement assistance, making it possible for you to align the program with your personal timeline and career goals.
-
After completing the CRA Certification in Texas, professionals can expect significant salary increases. Entry-level CRAs with certification can earn between $70,000 and $85,000, a 30% to 50% increase from uncertified roles. With experience, a certified CRA can move up to $100,000 or more in senior roles, such as Senior CRA or Clinical Trial Manager. The salary jump is especially prominent in trial hubs like Houston, Austin, and Dallas, where clinical research is growing rapidly. Certification ensures you have the skills and credibility needed to access these higher-paying positions and more prestigious companies.
-
No, you do not need prior clinical research experience to pursue CRA Certification in Texas. The certification program is designed to accommodate professionals who are new to the field. It provides comprehensive training on trial monitoring, GCP compliance, regulatory documentation, and site management, equipping you with the skills required to succeed as a CRA. However, if you already have some experience in clinical research or a related field, the program can serve as a valuable credential to enhance your qualifications and advance your career.
-
The job prospects for Clinical Research Associates (CRAs) in Texas are robust, with a growing demand for qualified professionals. Employers in cities like Houston, Dallas, and Austin are actively seeking certified CRAs to fill roles in Phase I–III trials, remote monitoring, and clinical project management. Companies such as Pfizer, Labcorp, Covance, and Baylor College of Medicine are major employers, offering competitive salaries and career growth opportunities. With the right certification, you can also explore roles with research institutions, pharmaceutical companies, and Contract Research Organizations (CROs) that value accredited training. The certification helps candidates stand out in a competitive job market and secure well-compensated roles.