Clinical Research Coordinator Certification in Zurich (Switzerland): Everything You Need to Know in 2025-2026
Becoming a Clinical Research Coordinator (CRC) in Zurich without certification in 2025–2026 is like applying to Swiss banks without financial credentials — you simply don’t stand a chance. Top CROs and sponsor-backed hospitals in Switzerland now require certification proof before even shortlisting. This isn’t just about “knowing GCP”; it’s about being legally and clinically trusted to coordinate multi-million-dollar trials that comply with Swissmedic, ICH-GCP, and EU Regulation 536/2014. Whether you're aiming to enter the clinical field or already assisting trials, unaccredited backgrounds are fast being filtered out.
What makes CRC certification even more critical in Zurich is the direct link between credentials and pay scale. Entry-level certified CRCs start between CHF 68,000–78,000, while uncertified assistants are often stuck at CHF 52,000–58,000 even with experience. The gap widens as trial complexity increases — oncology, medical devices, or multi-site EU trials offer premium roles only to those with certified expertise in regulatory handling, patient compliance, AE reporting, and protocol execution. This is not just career entry — it’s the fastest route to a 6-figure income in clinical research without a PhD or medical license.
What Is Clinical Research Coordinator Certification in Zurich Exactly? Skills Required and Jobs Explained
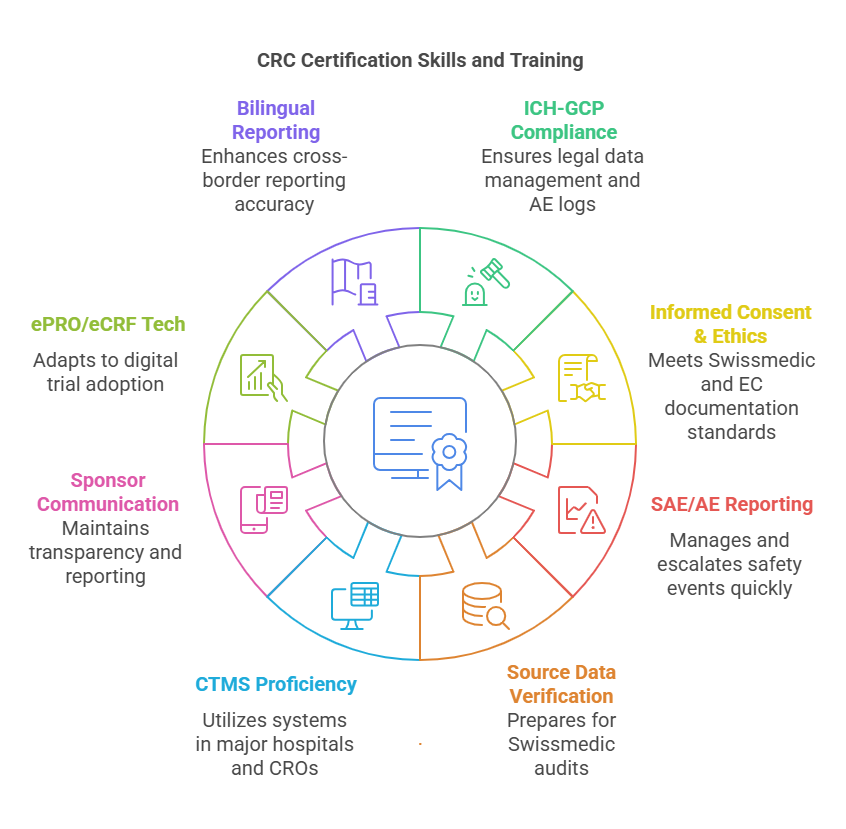
Clinical Research Coordinator (CRC) Certification in Zurich is a formal validation that you’re trained to independently manage, coordinate, and monitor clinical trials in compliance with Swissmedic guidelines, ICH-GCP protocols, and sponsor SOPs. Zurich’s biomedical and pharmaceutical sectors — driven by global CROs, hospital-based research centers like University Hospital Zurich, and biotech incubators in the Greater Zurich Area — are laser-focused on regulatory risk minimization, making certification non-negotiable.
This certification ensures you have operational-level proficiency in trial start-up, patient recruitment, data documentation, safety monitoring, and protocol adherence — all while maintaining ethical and regulatory compliance. In 2025–2026, with Switzerland’s expanding participation in decentralized and device-based trials, CRCs are also expected to assist with eSource data integration, remote patient monitoring tools, and trial-specific apps.
Why Should You Get CRC Certification to Work in Zurich?
In Zurich’s current clinical research environment, certification is the baseline filter for serious hiring. Sponsors and CROs now expect CRCs to prove regulatory compliance through credentials, not just job titles. Without certification, you're either underpaid or locked out of Phase II/III trial work entirely. Certified CRCs are fast-tracked into roles with direct sponsor interaction, multi-site coordination, and regulatory reporting authority — privileges that directly impact salary, title, and promotion speed. By 2025, non-certified profiles are increasingly being outsourced or contract-capped, while certified professionals are slotted into permanent, full-salary roles with CHF 20K+ higher median earnings.
| Aspect | Without CRC Certification | With CRC Certification |
|---|---|---|
| Starting Salary (Annual) | CHF 52,000 – CHF 58,000 | CHF 68,000 – CHF 78,000 |
| Eligible Trial Phases | Mostly Phase I or observational trials | Phase II, III, post-marketing trials |
| Role in Sponsor Communications | Indirect via site lead | Direct updates, report submissions |
| Job Security / Permanency | Project-based contracts only | Permanent or long-term roles preferred |
| Promotion Timeline | 3–5 years to CRA or PM roles | 1–2 years to CRA, CTM, or Line Manager |
Which Certification Should You Choose to Become a Clinical Research Coordinator in Zurich?
Switzerland’s clinical research ecosystem accepts a wide range of international CRC certifications, but only a few are optimized for Zurich-specific regulations, Swissmedic audits, and EU-GMP-aligned clinical standards. Programs from SOCRA or ACRP are globally recognized, but they’re costly, require eligibility verification, and often focus on U.S. FDA frameworks, which do not fully translate to Zurich’s multi-regulatory trial environment. Similarly, some local university-linked diplomas offer foundational clinical knowledge, but lack structured GCP protocol coverage or hands-on trial management simulation.
That’s where CCRPS (Certified Clinical Research Professionals Society) stands out. Their CRC Certification is fully self-paced, built around Swissmedic + ICH-GCP protocol training, and offers optional bootcamp acceleration. The curriculum is massively detailed, with 200+ lessons tailored to patient documentation, audit readiness, adverse event reporting, and real-world eCRF navigation. You don’t just get certified — you’re trained like a site-ready, sponsor-facing CRC from day one.
| Criteria | Other CRC Certifications | CCRPS CRC Certification |
|---|---|---|
| Accreditation | SOCRA/ACRP (U.S.-centric) | CPD + CME + ACCRE (International + EU-compatible) |
| Curriculum Depth | 80–120 lessons | 200+ modules, includes site + sponsor task simulations |
| Pacing Options | Fixed deadlines or live batches | 100% Self-paced + optional live Bootcamp |
| Tuition Flexibility | Upfront full payment only | Interest-free plans available |
| Instructor Access | General support team | Expert mentors with 10+ years clinical trial experience |
| Transparency | Instructor bios often not listed | Full team & syllabus visible before enrollment |
Why CCRPS’s Clinical Research Coordinator Certification Will Be a Game Changer for Your Career in Zurich
In Zurich’s fiercely competitive clinical research market, CRC certification from CCRPS isn’t just a title upgrade — it’s a salary and responsibility accelerator. Certified CRCs are trusted to handle Swissmedic audits, maintain direct contact with sponsors, and manage high-stakes trial logistics that non-certified staff are not legally cleared to oversee. This elevated scope leads to immediate salary bumps, contract-to-permanent conversions, and early promotion to CRA, CTM, or site lead roles.
Summarizing All You Need to Know About Getting Your Clinical Research Coordinator Certification in Zurich
Zurich’s clinical research sector is evolving fast — driven by EU-aligned trial mandates, digital health integration, and high-profile sponsor oversight. In this climate, getting certified as a Clinical Research Coordinator is no longer optional. It’s the minimum requirement to access real authority, salary growth, and long-term career mobility. CCRPS’s CRC Certification stands out for one reason: it prepares you not just to enter the job, but to excel from day one in Switzerland’s strict, sponsor-governed research ecosystem.
| Topic | Details for Zurich (2025–2026) |
|---|---|
| Is CRC Certification Required? | Strongly preferred for all sponsor-facing roles; often mandated by CROs and university hospitals |
| Top Hiring Locations | University Hospital Zurich, Roche, Novartis sites, CRO hubs in greater Zurich area |
| Recommended Certification | CCRPS Clinical Research Coordinator Certification (CPD, CME, ACCRE-accredited) |
| Average Salary Increase | CHF 16,000–28,000 more annually after certification |
| Learning Mode | Self-paced online + optional Bootcamp for real-world case training |
| Job Titles Post-Certification | CRC, Site Coordinator, Trial Monitor, Lead CRA, GCP Compliance Officer |
Frequently Asked Questions
-
While not legally mandatory, most employers in Zurich will not hire you for CRC roles without certification. Hospitals like Universitätsspital Zürich and CROs such as ICON, IQVIA, and Labcorp require certified proof that you're trained in Swissmedic guidelines, ICH-GCP protocols, and sponsor-facing responsibilities. Even when not required by law, it's often listed as a “required qualification” in real job postings. Without it, you're usually limited to temporary assistant roles or low-trust positions with no authority over trial operations or documentation.
-
Most candidates complete CCRPS’s Clinical Research Coordinator Certification in 4 to 8 weeks depending on pace. The course is 100% self-paced, meaning you can move through the 200+ lessons quickly if you're experienced — or take time if you're new. You can also opt for live Bootcamp-style mentorship for faster progress. It’s designed to be flexible without compromising regulatory depth — covering GCP, AE documentation, Swissmedic prep, eCRF workflows, and trial tech tools in one program.
-
Yes, but with limitations. Certifications like SOCRA or ACRP are globally respected, but often emphasize FDA frameworks, which don’t fully align with Swissmedic or EU Regulation 536/2014. Many Zurich employers now prefer certifications tailored to ICH-GCP in a Swiss/EU context, such as CCRPS’s internationally accredited CRC certification. If your training doesn't include European protocols, you may be required to complete additional onboarding or shadowing before getting full CRC responsibilities.
-
CCRPS offers career support resources, 1-on-1 mentorship during training, resume building guidance, and connections to a global recruiter network — but does not guarantee job placement. In Zurich, however, certified CCRPS graduates often find opportunities through university hospitals, research foundations, and CROs, since the program’s accreditation (CPD, CME, ACCRE) and curriculum are recognized by EU-based hiring teams. Most learners find the certification itself removes 70% of hiring barriers in Switzerland’s research sector.
-
A CRC (Clinical Research Coordinator) manages trials onsite, coordinating between the PI, patients, and sponsors. A CRA (Clinical Research Associate) monitors sites across multiple locations and ensures protocol and data compliance. Most Zurich-based CRCs use certification as a stepping stone to become CRAs within 1–2 years. If you're just starting out, CRC is the logical entry — especially since CRA roles require prior hands-on site experience, which CRC roles provide.