Clinical Research Coordinator (CRC) Role: Essential Skills & Responsibilities
In today’s clinical trial landscape, the Clinical Research Coordinator (CRC) is no longer just a behind-the-scenes technician—they are the operational backbone of human research. From patient screening and informed consent to data entry and adverse event tracking, CRCs are the driving force that keeps trials ethically sound, protocol-compliant, and audit-ready. Their role sits at the intersection of regulatory precision, real-time patient care, and sponsor expectations—making them indispensable in both academic medical centers and private research sites.
The modern CRC wears multiple hats: regulatory liaison, data custodian, subject advocate, and site operations expert. With ICH-GCP, FDA 21 CFR Part 11, and evolving sponsor protocols demanding tighter oversight, today’s CRC must master not only the science but also the systems—CTMS platforms, eTMF filing, and digital EDC tools. The future of compliant, efficient, and patient-safe clinical research runs through the skillset of the CRC. Whether coordinating a phase I oncology study or a multi-center vaccine trial, CRCs are the quiet force delivering reproducibility, subject safety, and protocol fidelity—every day, every visit, every trial.
Who Is a Clinical Research Coordinator?
Site-Level vs Sponsor-Level CRCs
While most Clinical Research Coordinators operate at the site level, managing participant interaction and protocol execution, a growing number are now embedded within sponsor or CRO teams. Site-level CRCs are the frontline operators—handling subject recruitment, data entry, and visit coordination. They work closely with Principal Investigators (PIs) and patients, ensuring protocol adherence in real-time. In contrast, sponsor-level CRCs are more likely to support remote trial monitoring, documentation standardization, and broader operational strategy across multiple trial sites.
Both roles demand a firm grasp of regulatory standards, trial workflows, and communication fluency, but the context and pressure points vary significantly. Site CRCs must master patient-facing dynamics and real-time documentation accuracy. Sponsor CRCs, on the other hand, focus more on cross-site consistency, training, and internal audit preparedness.
Regulatory, Operational, and Ethical Scope
The CRC’s role is governed by a tightly integrated matrix of regulatory, operational, and ethical mandates—and failing in any domain can derail a trial. On the regulatory front, CRCs are the guardians of informed consent forms (ICFs), ensuring each participant receives the correct version, signs it appropriately, and is re-consented if protocol amendments arise. This process must follow IRB guidelines and be traceable for FDA inspection.
Operationally, CRCs are responsible for protocol execution at the site level. This includes ensuring inclusion/exclusion criteria are applied without deviation, coordinating patient visits, managing sample collection logistics, and entering data into electronic data capture (EDC) systems. Each action must align with the sponsor’s case report forms (CRFs) and timeline expectations.
Ethically, CRCs protect subject welfare. This includes documenting and escalating adverse events (AEs) and serious adverse events (SAEs) in a timely and complete manner. It also means preserving privacy, ensuring voluntary participation, and identifying protocol deviations that may impact subject safety or data integrity.
In short, a CRC isn’t just a project coordinator—they are the quality control engine, ethics guardian, and trial execution expert embedded directly within the research pipeline. Their meticulous work is what allows clinical data to withstand regulatory scrutiny and ultimately contribute to global medical advancements.
Key Responsibilities of a CRC in 2025
Study Start-Up Duties
The study start-up phase is one of the most regulatory-intensive periods in a clinical trial, and the CRC is at the center of it. Before a single subject can be enrolled, the CRC ensures that IRB submissions are complete, up-to-date, and include all required supporting documentation—protocols, investigator brochures, ICFs, and recruitment materials. The CRC coordinates directly with the sponsor or CRO to resolve any IRB queries quickly, preventing delays.
At the site level, CRCs are responsible for clinical trial binder setup, preparation of site standard operating procedures (SOPs), and staff training logs. Equally critical is the informed consent process—the CRC verifies that the correct ICF version is being used, that all required signatures are captured, and that re-consents are properly tracked after protocol amendments.
This phase also demands setup of delegation logs, confirmation of investigational product (IP) receipt, and verifying lab certifications are valid for GCP compliance. CRCs handle pre-study visits with monitors, ensure site readiness, and often troubleshoot last-minute sponsor requirements—setting the tone for trial efficiency and quality.
Ongoing Trial Management
Once enrollment begins, the CRC’s role shifts to continuous operations management. The CRC coordinates subject visits to align with protocol windows, schedules labs and imaging, and ensures source documentation supports all case report form (CRF) entries. Visit windows must be strictly followed to prevent protocol deviations, and all procedures—vital signs, ECGs, sample collections—must match the protocol.
CRCs enter real-time data into electronic data capture (EDC) systems, handling queries raised by sponsors or monitors swiftly. Each data point must be cross-verified with source notes and hospital systems. When study monitors conduct site monitoring visits (SMVs), CRCs provide access to eTMF documents, verify visit logs, and clarify discrepancies without delay.
CRCs are also the point of contact for SAE reporting, lab abnormalities, and drug accountability. They ensure compliance with investigational product handling, monitor protocol deviations, and maintain communication logs between PI, sponsor, and IRB—all while safeguarding the subject’s rights and privacy.
Close-Out and Audit Support
At the trial’s end, the CRC is responsible for meticulous documentation reconciliation and regulatory close-out activities. All essential documents must be finalized and filed within the electronic trial master file (eTMF) or physical binders, depending on sponsor preference. The CRC also ensures that all monitor queries are resolved and data queries closed in the EDC.
This stage includes archiving of documentation, reconciling drug inventory, and confirming that sample shipments are complete. In audits—whether from the sponsor, IRB, or regulatory authorities—the CRC is often the person walking the auditor through site documentation, SOP compliance, and informed consent records.
The final weeks of a trial can trigger surge workloads with backlogged queries, missing lab results, or overdue monitoring follow-ups. A skilled CRC closes every loop, ensuring the site exits cleanly, compliantly, and without unresolved deviations that could jeopardize data integrity or subject safety.
Technical Skills Every CRC Must Master
eTMF and CTMS Systems
Modern clinical trials generate thousands of documents, and managing them accurately is no longer optional—it’s mission-critical. CRCs must be proficient with electronic Trial Master File (eTMF) systems like Veeva Vault, Florence, or Trial Interactive. These platforms store essential regulatory documents, delegation logs, protocol amendments, IRB approvals, and ICF versions—every item that an auditor will scrutinize.
Beyond document control, Clinical Trial Management Systems (CTMS) like Medidata, MasterControl, or Oracle Siebel help CRCs track visits, manage budgets, schedule monitoring activities, and oversee subject enrollment status. The CRC must know how to generate reports, update subject progress, and manage sponsor or CRO queries within these platforms without delay. A single lapse in CTMS updates can create serious data integrity gaps or misalignment between the site and sponsor timelines.
Mastery of these tools isn't about checkbox compliance—it's about creating real-time visibility across the study and ensuring the trial can stand up to any inspection or deviation review.
GCP and 21 CFR Part 11 Compliance
Every action a CRC takes must align with Good Clinical Practice (GCP) guidelines and meet the expectations of FDA 21 CFR Part 11 for electronic systems. GCP training is not just a formality—it’s the operating manual for every interaction involving human subjects, investigational products, and sponsor protocols. CRCs must be fluent in core GCP principles: subject protection, data reliability, and document traceability.
Equally important is understanding 21 CFR Part 11, which governs the use of electronic signatures, audit trails, and system validations. CRCs working in electronic platforms like EDC, CTMS, and eTMF must ensure that user access, data edits, and sign-off procedures follow regulatory expectations. A CRC who cannot confidently navigate these compliance layers can become a risk factor in both audits and trial execution.
Sponsors increasingly favor sites whose CRCs can demonstrate documentation rigor and audit-readiness in both digital and hybrid environments.
Lab and Diagnostic Data Management
A high-performing CRC must also be proficient in managing clinical lab reports, radiology findings, and diagnostic test data. This includes tracking lab kits, ensuring chain-of-custody for biospecimens, and resolving out-of-range lab values or flagged imaging findings. These tasks require more than clerical oversight—they demand medical fluency, especially when AE or SAE classifications depend on clinical interpretation.
CRCs must also maintain alignment between lab results and CRF entries, escalate abnormalities to the PI, and ensure that all lab documentation is archived and version-controlled. In multisite studies, this skill becomes critical for harmonizing lab workflows across central and local labs while staying within protocol parameters.
| Skill Area | Tools/Concepts Involved | Purpose & Impact |
|---|---|---|
| eTMF & CTMS Systems | Veeva Vault, Florence, Trial Interactive, Medidata, Oracle Siebel | Document control, visit tracking, query resolution, audit preparedness |
| GCP & 21 CFR Part 11 Compliance | GCP guidelines, electronic signatures, audit trails, system validation | Ensures regulatory compliance, protects subject data, supports inspections |
| Lab & Diagnostic Data Management | Lab kits, biospecimen tracking, radiology reports, AE/SAE classification | Maintains clinical accuracy, aligns labs with CRFs, flags safety issues |
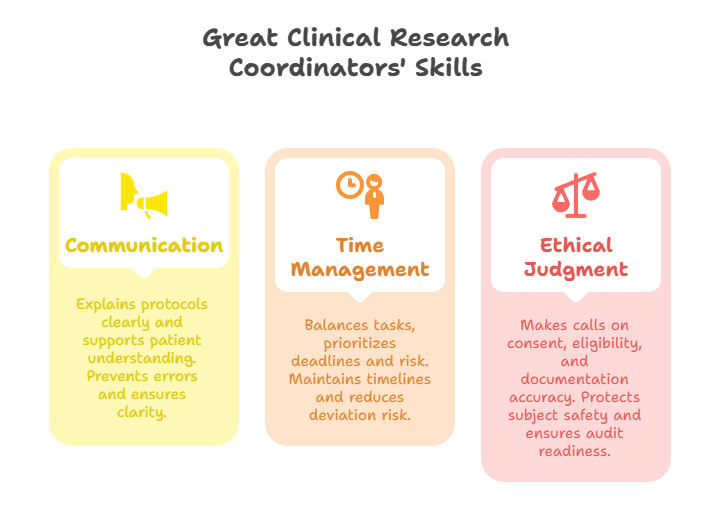
Soft Skills That Separate Good from Great Coordinators
Communication with PI, Monitors, and Patients
The best CRCs aren’t just operationally precise—they are exceptional communicators. This means knowing how to translate complex protocol changes to Principal Investigators (PIs), respond to monitoring queries with clarity and completeness, and support patient understanding during informed consent. At any moment, the CRC is the communication nexus for the trial.
Effective communication also means documenting interactions clearly and maintaining open dialogue with sponsors, CROs, and IRBs. Missed or miscommunicated updates can result in protocol violations or delayed regulatory approvals. Great CRCs anticipate questions, prepare concise summaries, and ensure no ambiguity in site documentation or data interpretation.
Time and Stress Management
CRC workflows are unrelenting—there are always labs to track, CRFs to update, monitors to host, and visits to prepare within tight protocol windows. What separates the high performers is their ability to manage time under pressure while keeping every task accurate and audit-ready.
CRCs must prioritize by risk and deadline—handling SAE reporting within 24 hours while still preparing for SIVs or reconciling source documentation. Stress management isn’t about avoidance—it’s about building repeatable systems, using scheduling tools, and setting expectations with study staff and PIs.
Those who thrive use their workflow discipline as a competitive edge, preventing small backlogs from snowballing into regulatory risk or patient delays.
Ethical Judgment and Precision
Clinical trials demand uncompromising ethics, and CRCs sit on the front lines of those decisions. Whether assessing whether a patient truly meets inclusion criteria, flagging a PI's missed signature, or identifying consent irregularities, ethical clarity must guide every CRC action.
Great CRCs also exhibit micro-level precision. They spot version mismatches in ICFs, catch deviations in drug logs, and know when a note-to-file isn’t enough. Their judgment ensures that the trial not only runs smoothly—but that its data can withstand global regulatory scrutiny and protect participant safety to the highest standard.
Common Career Pathways for CRCs After Certification
Clinical Team Lead, CRA, Trial Manager, QA Auditor
Once certified, skilled CRCs become highly visible to sponsors, CROs, and regulatory agencies—not just as operational staff, but as leadership-track professionals. The first natural step for many is the Clinical Team Lead (CTL) role. Here, former CRCs supervise junior coordinators, manage site timelines, and escalate operational risks to sponsors while maintaining subject safety and documentation integrity across studies.
Many CRCs also transition into Clinical Research Associate (CRA) roles, particularly if they’ve mastered site management, monitoring preparation, and sponsor communication. Their deep knowledge of site-level documentation, GCP workflows, and eTMF navigation gives them an edge over those entering from academic or non-clinical tracks.
For those with an interest in operations or strategic oversight, moving into Trial Manager or Clinical Project Manager roles becomes a viable pathway. These professionals coordinate timelines, budgets, vendor relationships, and regulatory submission strategies across multiple trials and geographies.
Another key route is Quality Assurance (QA) auditing. CRCs who have developed expertise in SOP compliance, audit trail verification, and 21 CFR Part 11 protocols often move into internal QA roles at CROs or sponsor companies. They review site documentation, conduct audit visits, and lead CAPA development.
What unites all these paths is one trait: high-performing CRCs understand trial execution from the ground up. They don’t just follow checklists—they lead with data integrity, protocol expertise, and investigator trust. That’s why sponsors continue to view certified CRCs as a talent pipeline for leadership in global clinical research operations.
| Career Path | Role Description | Core Skills Transitioned From CRC Role |
|---|---|---|
| Clinical Team Lead (CTL) | Supervises CRCs, manages timelines, escalates site-level issues | Team coordination, protocol execution, documentation oversight |
| Clinical Research Associate (CRA) | Monitors multiple sites, ensures data quality, supports sponsor inspections | Site management, source verification, GCP compliance |
| Trial/Project Manager | Oversees trial operations, budget, vendor management, cross-functional planning | Strategic planning, regulatory tracking, cross-site coordination |
| Quality Assurance Auditor | Conducts internal audits, verifies SOP adherence, leads CAPA responses | Audit prep, documentation control, 21 CFR Part 11 understanding |
How the ACRCC Certification Prepares You for Real-World CRC Roles
The ACRCC – Clinical Research Coordinator Certification by CCRPS isn’t just a certificate—it’s a deep-practical training platform built specifically for coordinators who want to excel at the site level and beyond. With over 100 industry-aligned modules, the course is designed to give aspiring CRCs the technical fluency and regulatory confidence expected by sponsors, CROs, and regulatory bodies alike.
One of its standout features is hands-on training in CTMS and eTMF systems, which are essential for site-level operations, document control, and inspection readiness. Learners work through simulated workflows involving trial startup documents, site visit coordination, and close-out documentation—all framed in a real clinical setting, not academic abstraction.
The certification also prepares CRCs for GCP audits, SAE reporting, and informed consent management—with lessons rooted in FDA expectations and ICH guidelines. Each module integrates real-world case simulations, helping learners spot protocol deviations, document discrepancies, and compliance gaps in advance.
For CRCs aiming to move into CRA, Trial Manager, or QA roles, the ACRCC program lays the foundational groundwork. By covering topics like regulatory filing structure, audit readiness, and cross-functional communication, it ensures graduates can operate beyond data entry and visit scheduling—they’re trained for operational leadership.
Whether you’re a new entrant to the field or a site staffer ready to move up, ACRCC certification signals to sponsors and employers that you’re trained, tested, and ready to deliver protocol fidelity and subject safety under pressure.
Frequently Asked Questions
-
Most CRCs begin with a bachelor’s degree in life sciences, healthcare, or a related field, but certification is what sets serious candidates apart. Employers increasingly prioritize candidates with a recognized credential like the ACRCC Certification from CCRPS, which proves competency in GCP, ICF handling, AE reporting, and eTMF/CTMS systems. Some roles require prior site experience or internships in research, but many positions accept entry-level applicants who have demonstrated protocol literacy, regulatory readiness, and EDC fluency through certification programs. Clinical experience—whether as a nurse, phlebotomist, or medical scribe—also translates well to CRC duties. A combination of academic foundation and regulatory-aligned certification offers the most direct route into the role.
-
The Clinical Research Coordinator role is a hybrid position that demands both administrative precision and clinical awareness. On the administrative side, CRCs manage IRB documentation, ICF versions, source document filing, and eTMF control. On the clinical side, they coordinate subject visits, monitor lab results, and often observe patient interactions directly. CRCs don’t typically perform medical procedures, but they must understand them in order to ensure protocol adherence and accurate CRF entries. Their success depends on their ability to interpret lab data, manage AE documentation, and act as the liaison between sponsors, monitors, and clinical staff. It's one of the few roles where operational detail and clinical literacy merge daily.
-
In 2025, entry-level CRCs earn between $52,000 and $68,000 annually in the U.S., depending on location and setting (academic site vs private site). Mid-level CRCs with 3–5 years of experience and ACRCC certification often earn $70,000 to $85,000, particularly at oncology or high-enrollment sites. Senior CRCs who supervise staff, lead multicenter studies, or specialize in audit readiness can earn upwards of $95,000. In high-cost regions or major pharma-affiliated centers, total compensation may also include bonuses tied to subject enrollment and retention metrics. CRCs with eTMF, CTMS, and GCP expertise are especially competitive in CRO and sponsor environments.
-
A CRC (Clinical Research Coordinator) works at the site level and is directly involved in patient-facing trial execution. They manage visits, enter EDC data, track labs, and ensure protocol compliance on-site. A CRA (Clinical Research Associate), in contrast, monitors multiple sites on behalf of a sponsor or CRO. CRAs travel or work remotely to verify source data, check compliance, and assess site documentation and operations. While CRCs are embedded in site operations, CRAs function as external auditors. Many CRAs begin their careers as CRCs—the hands-on experience gives them deep insight into site-level workflows, which is invaluable when assessing site performance and regulatory alignment.
-
Yes, AE and SAE reporting is one of the CRC’s core responsibilities. CRCs document patient symptoms, lab abnormalities, and clinical findings that meet the criteria for adverse events under the protocol or GCP definitions. Once identified, they must report the event to the sponsor or CRO, often through the EDC or SAE system, and ensure that IRB and PI notifications are completed on time. For serious adverse events (SAEs), CRCs are expected to submit the full documentation—lab values, hospital records, narratives—within 24–48 hours. They also help reconcile AE data with CRFs, ensuring data accuracy and regulatory compliance across multiple systems.
-
No prior clinical research experience is required to take the ACRCC Certification by CCRPS. The course is structured to support both entry-level professionals and current site staff. It includes over 100 lessons covering eTMF handling, CTMS navigation, GCP principles, SAE documentation, and informed consent workflows. Real-world simulations prepare you for actual sponsor queries, audit conditions, and patient visit timelines. The program is designed for immediate career application, helping even those from non-research backgrounds transition into CRC roles with confidence. However, pairing the certification with a research internship or assistantship can strengthen job prospects, especially in academic medical centers.
-
CRCs should be fluent in electronic Trial Master File (eTMF) systems like Veeva Vault or Florence, as well as Clinical Trial Management Systems (CTMS) such as Medidata or Oracle Siebel. These platforms are central to document control, regulatory filing, and site activity tracking. In addition, CRCs must be skilled in Electronic Data Capture (EDC) systems like Medrio, Rave, or REDCap. They should also understand SAE reporting platforms and lab management portals specific to central lab vendors. Familiarity with 21 CFR Part 11 compliance standards for all digital systems is a must. Mastery of these tools ensures audit readiness and operational efficiency at all trial stages.
-
Absolutely. The CRC role is often the gateway to higher-tier clinical research positions. After gaining experience, many CRCs move into roles such as Clinical Research Associate (CRA), Trial Manager, or Quality Assurance Auditor. Others advance into Regulatory Affairs, Medical Affairs, or Clinical Operations leadership roles. The foundation in GCP, protocol execution, and documentation makes CRCs highly desirable for sponsors and CROs looking for internal hires. Certification through programs like ACRCC also signals that a coordinator is ready for greater responsibility. The more systems, audits, and therapeutic areas a CRC handles, the faster their career trajectory accelerates.
Final Thoughts
The Clinical Research Coordinator is not just a support role—it’s the operational nerve center of every clinical trial. From pre-study start-up to final audit, CRCs manage the delicate balance of ethics, precision, and speed that determines whether a study succeeds or stalls. In 2025, the demand for CRCs who can confidently navigate eTMF systems, GCP audits, AE reporting, and patient coordination is higher than ever.
Whether you're entering clinical research for the first time or scaling up for leadership, mastering the CRC skill set makes you indispensable across all trial phases—from early feasibility to final FDA submission. Certification programs like the ACRCC by CCRPS offer more than knowledge—they provide real-world workflows, sponsor expectations, and the documentation mastery you need to lead confidently.
In a field driven by compliance, data integrity, and patient safety, the CRC is the one who ensures all three align—visit after visit, trial after trial. This role is not just a stepping stone; it's a launchpad into the heart of global research innovation.
Poll: Which career path are you most interested in after becoming a CRC?