Clinical Trial Associate vs Clinical Research Associate
The Battle of CTAs vs. CRAs: Which One Wins in the Clinical Research Arena?
Ever wondered what the difference is between a Clinical Trial Associate (CTA) and a Clinical Research Associate (CRA)? You’re not alone. It’s like comparing Batman and Iron Man—both are superheroes, but their gadgets and missions differ. Whether you're planning a career in clinical research or just want to impress your colleagues with your industry knowledge, this guide will break down the roles, responsibilities, and career opportunities in a way that even your non-science friends would understand. Let’s get started!
What is a Clinical Trial Associate (CTA)?
A Clinical Trial Associate (CTA) is the unsung hero of clinical trials, keeping everything running behind the scenes. If clinical research were a Broadway show, CTAs would be the stage managers, making sure everything is in place for a flawless performance.
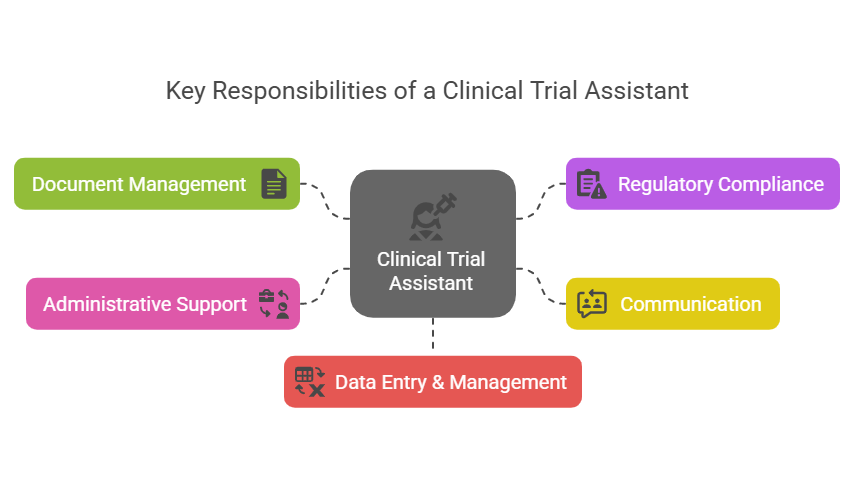
Key Responsibilities of a CTA
Document Management: Organizing and maintaining Trial Master Files (TMFs) to meet regulatory requirements.
Regulatory Compliance: Assisting with Institutional Review Board (IRB) submissions and ensuring adherence to guidelines.
Communication: Acting as a bridge between stakeholders, study sites, and sponsors.
Administrative Support: Managing logistics, from setting up meetings to arranging site visits.
Data Entry & Management: Ensuring trial data is accurately recorded and stored.
Skills & Qualifications
Bachelor’s degree in life sciences, healthcare, or a related field.
Meticulous attention to detail and organizational skills.
Strong communication and problem-solving abilities.
Proficiency in clinical trial management systems (CTMS) and regulatory requirements.
What is a Clinical Research Associate (CRA)?
A Clinical Research Associate (CRA) is like a field agent, constantly on the move to ensure clinical trials follow the rules and produce reliable data.
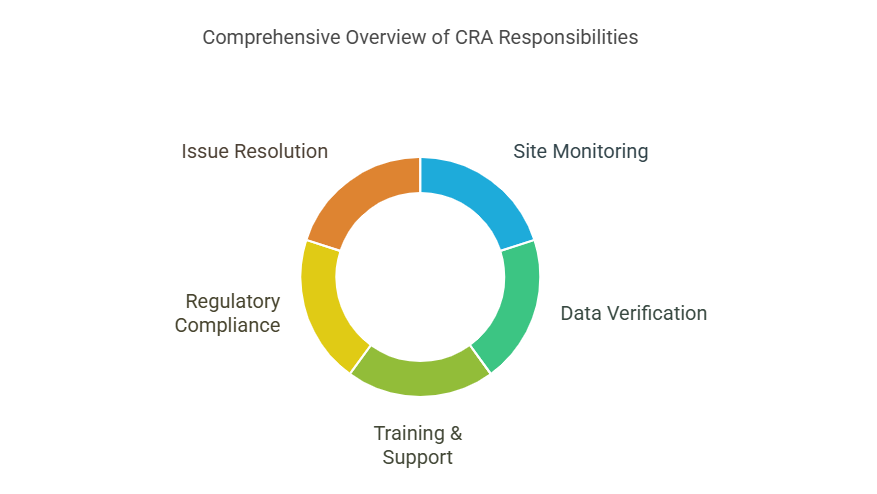
Key Responsibilities of a CRA
Site Monitoring: Conducting routine visits to trial sites to ensure compliance with protocols.
Data Verification: Ensuring that the collected data is accurate and complete.
Training & Support: Educating site staff about trial procedures and protocols.
Regulatory Compliance: Preparing for audits and ensuring adherence to Good Clinical Practice (GCP).
Issue Resolution: Identifying and addressing discrepancies or protocol violations.
Skills & Qualifications
Bachelor's degree in life sciences, nursing, or pharmacy.
Prior experience in clinical research is preferred.
Strong analytical skills and attention to detail.
Willingness to travel frequently.
The clinical research industry offers diverse career paths, from trial management to regulatory oversight. Understanding the scope of clinical research can help professionals choose the right role based on their skills and interests.
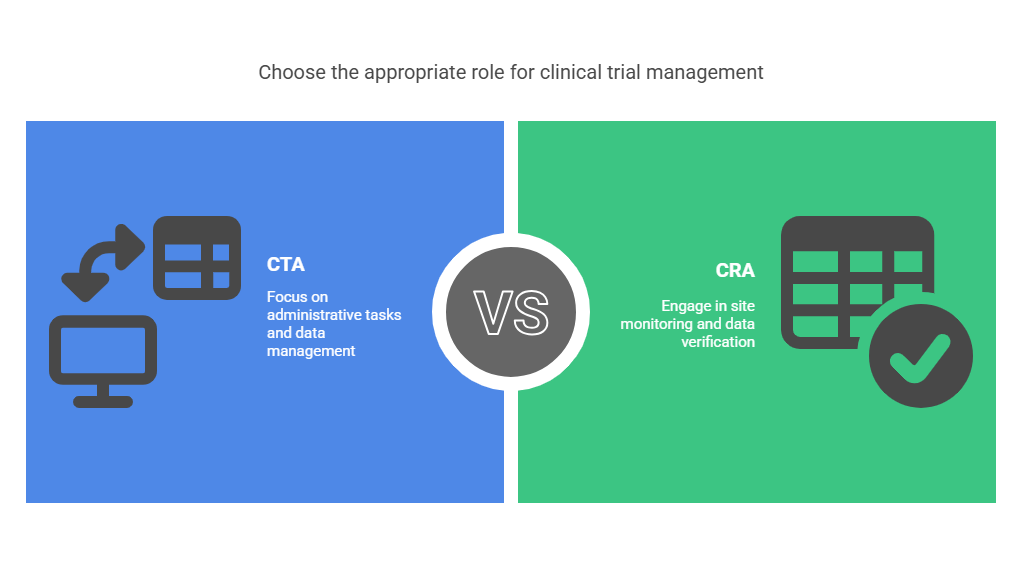
Differences Between CTAs and CRAs
Scope of Work
CTA: Focuses on administrative and regulatory tasks.
CRA: Involves hands-on site monitoring and compliance checks.
Data Handling
CTA: Manages and organizes trial data.
CRA: Verifies the accuracy and compliance of trial data.
Interaction with Study Sites
CTA: Limited direct contact.
CRA: Regular site visits and direct engagement.
Industry Trends in 2025 for Clinical Research Roles
The clinical research industry is evolving rapidly, with new technologies and methodologies reshaping traditional roles. In 2025, Clinical Trial Associates (CTAs) and Clinical Research Associates (CRAs) must adapt to several key trends:
1. Decentralized Clinical Trials (DCTs) Are the Future
The rise of remote and virtual trials means fewer site visits and more reliance on digital tools. While CRAs may travel less, they will need to become experts in eSource documentation, electronic patient-reported outcomes (ePROs), and remote monitoring systems. CTAs, on the other hand, will handle more digital administrative tasks like telemedicine coordination and AI-powered trial documentation.
2. Artificial Intelligence (AI) and Automation
AI is transforming data management in clinical trials. Automation tools are reducing the workload for CTAs, especially in document management, while CRAs benefit from AI-powered risk-based monitoring (RBM) that pinpoints trial risks faster than traditional methods.
3. Regulatory Changes Post-Pandemic
With regulatory agencies like the FDA, EMA, and MHRA continuously updating clinical trial guidelines, both CTAs and CRAs must stay current with compliance changes. Good Clinical Practice (GCP) training and continuous education will be crucial in 2025.
Career Growth: CTA vs. CRA – Which is the Better Long-Term Choice?
Choosing between a CTA and CRA career depends on your interests, skills, and career goals. Let’s compare the long-term career potential:
💡 CTA to CRA Transition Tip: If you’re a CTA and want to move into a CRA role, gaining a Good Clinical Practice (GCP) Certification and clinical trial monitoring experience can fast-track your progress. Aspiring Clinical Research Coordinators (CRCs) must develop key competencies such as regulatory compliance, data management, and patient interaction. These skills for clinical research coordinators are essential for excelling in the role and ensuring smooth trial execution.
10 Lesser-Known Facts About Clinical Research Roles
CTAs can transition into CRAs with experience and additional certifications (Source).
The average CRA travels 60% of the time (Source).
Pharmaceutical companies prefer CRAs with GCP certification (Source).
CTAs can become Clinical Project Managers after gaining experience (Source).
CRAs earn 20-30% more than CTAs on average (Source).
Most CTAs work in contract research organizations (CROs) (Source).
GCP violations can disqualify an entire study (Source).
Clinical trials are increasingly shifting to virtual models (Source).
The demand for CRAs is expected to grow by 10% by 2030 (Source).
AI is revolutionizing trial data management (Source).
Ready to Advance Your Clinical Research Career?
If you’re serious about excelling as a CTA or CRA, check out top industry certifications from CCRPS. They offer specialized courses, including:
Good Clinical Practice Certification
Clinical Research Associate Certification
Clinical Research Coordinator Certification
Research Assistant Training Program
Clinical Research Management Training
Pharmacovigilance Certification
Medical Monitor Certification
Principal Investigator Certification
These programs can help you stand out and accelerate your career in the evolving world of clinical research.
Final Thoughts: Choosing the Right Path in Clinical Research
Both Clinical Trial Associates (CTAs) and Clinical Research Associates (CRAs) play essential roles in clinical trials. Whether you prefer the structured, regulatory-focused work of a CTA or the dynamic, site-monitoring responsibilities of a CRA, the clinical research field offers exciting career growth and job security.
If you’re currently a CTA looking to transition into a CRA role, or a beginner wanting to break into the clinical research industry, certifications can set you apart. Courses like the Good Clinical Practice (GCP) Certification, Clinical Research Associate Certification, and Clinical Research Coordinator Certification offered by CCRPS can help you gain the necessary skills and qualifications.
Ready to advance your clinical research career? Explore CCRPS’s industry-leading training programs today!
-
CRAs typically earn 20-30% more than CTAs due to their direct involvement in site monitoring and compliance.
-
Yes, with experience and certifications like GCP, many CTAs transition into CRA roles.
-
Some CRA roles allow partial remote work, but site visits require travel.
-
Pharmaceutical companies, CROs, hospitals, and biotech firms frequently hire these professionals.
-
GCP, CCRA, and CRC certifications enhance job prospects.
-
With a bachelor’s degree and relevant experience, it can take 2-3 years.
-
They commonly use CTMS, electronic data capture (EDC) systems, and regulatory submission tools.
-
Regulatory compliance, data accuracy, and site management are the most significant challenges.