Common Mistakes During GCP Training and How to Avoid Them
Good Clinical Practice (GCP) is a set of international standards that ensures the integrity, safety, and quality of clinical trials. These guidelines are crucial for anyone involved in clinical research, especially those preparing for a GCP certification. However, many individuals make common mistakes during their GCP training, which can hinder their understanding and affect their performance in the certification exams. This blog will discuss the most frequent mistakes during GCP training and provide practical tips on how to avoid them.
Overlooking Key GCP Principles During Training
Good Clinical Practice (GCP) consists of several key principles that ensure the safety, rights, and well-being of clinical trial participants and the integrity of data. However, many trainees make the mistake of overlooking or skipping over these principles, focusing instead on other areas that seem more immediately relevant.
Related Blog: The Importance of GCP for Research Coordinators and Investigators
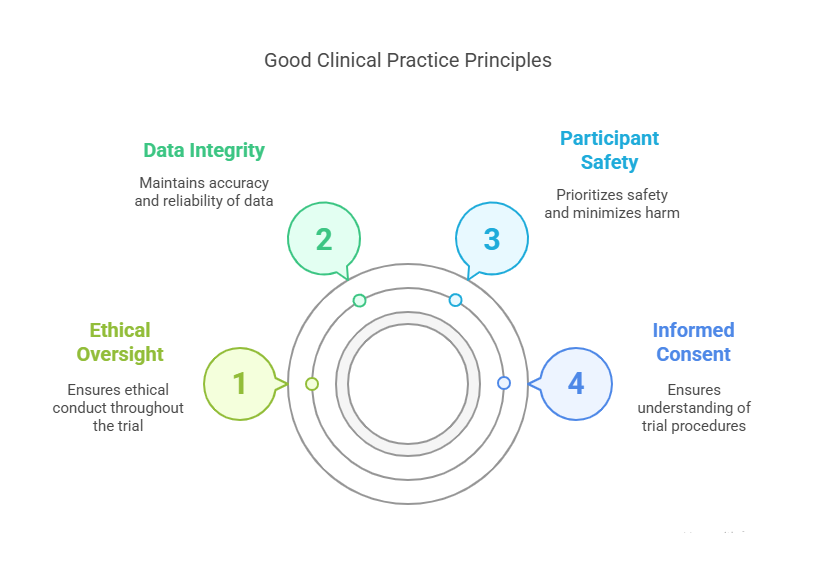
What Key Principles Should Be Focused On:
Informed Consent: Ensuring that participants fully understand the trial’s procedures, risks, and benefits before agreeing to participate.
Participant Safety: Always prioritizing the safety of the participant and minimizing any potential harm.
Data Integrity: Ensuring that the data collected during clinical trials is accurate, reliable, and documented correctly.
Ethical Oversight: Ethical principles must be adhered to in all stages of a trial, including proper oversight by an ethics committee or institutional review board (IRB).
By missing out on or not fully understanding these principles, you might miss key parts of the training that are essential for both conducting research and obtaining certification. Skipping over them could result in an incomplete understanding of GCP, which will hinder your performance in both your training and certification exams.
How to Avoid This Mistake:
Stay Comprehensive: Ensure you are not just reading but understanding the foundational principles. Engage with case studies and practical examples.
Ask for Clarification: If you feel confused about a specific principle, don't hesitate to ask instructors or peers for further explanation.
Apply Principles to Real-World Scenarios: Use practical examples and case studies to better understand how each principle plays a role in a clinical trial.
Not Taking Training Seriously Enough
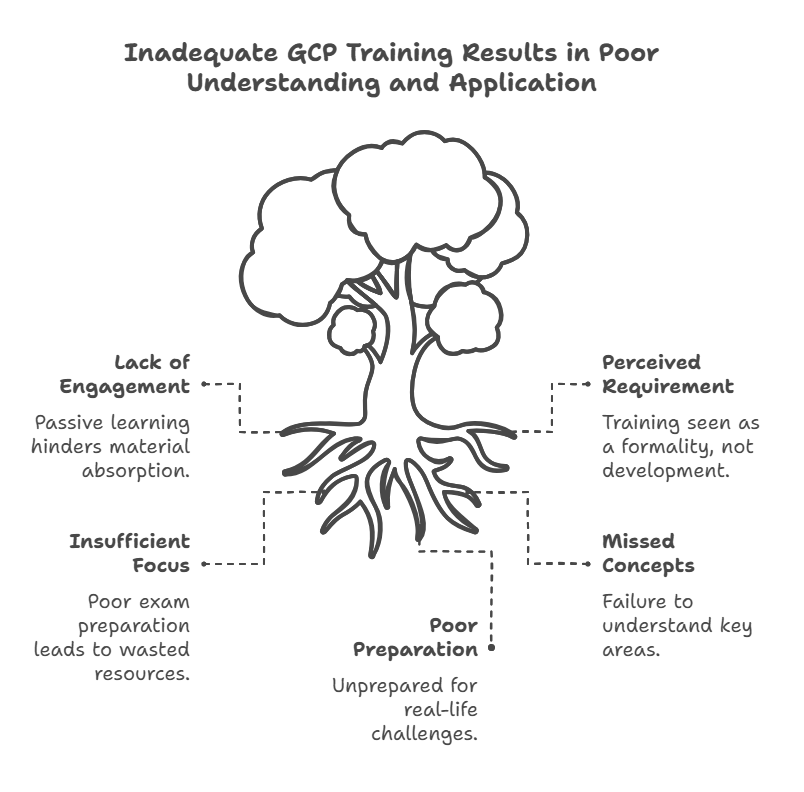
Some individuals may view GCP training as a mere requirement or a box to check rather than an essential part of their professional development. When this happens, training becomes a passive experience, and learners do not fully absorb the material. This could lead to poor understanding and, ultimately, failure during exams.
Consequences of Not Taking It Seriously:
Missed Concepts: You may fail to understand key areas that will be crucial when dealing with patients, trial data, and regulatory bodies.
Poor Exam Preparation: Insufficient focus during training may lead to low exam scores and wasted time and resources.
Difficulty in Real-World Application: GCP principles are vital when managing actual clinical trials, and without a serious approach, you may find yourself unprepared for real-life challenges.
How to Avoid This Mistake:
Treat It as a Priority: Treat GCP training as an important aspect of your career development. The knowledge gained will not only help you pass exams but also ensure that you can safely and efficiently conduct clinical trials.
Commit to Regular Study Sessions: Set aside dedicated time for study and avoid procrastination. Constant review is key to retaining the material.
Stay Engaged: Participate actively in class discussions, engage with the content, and take detailed notes.
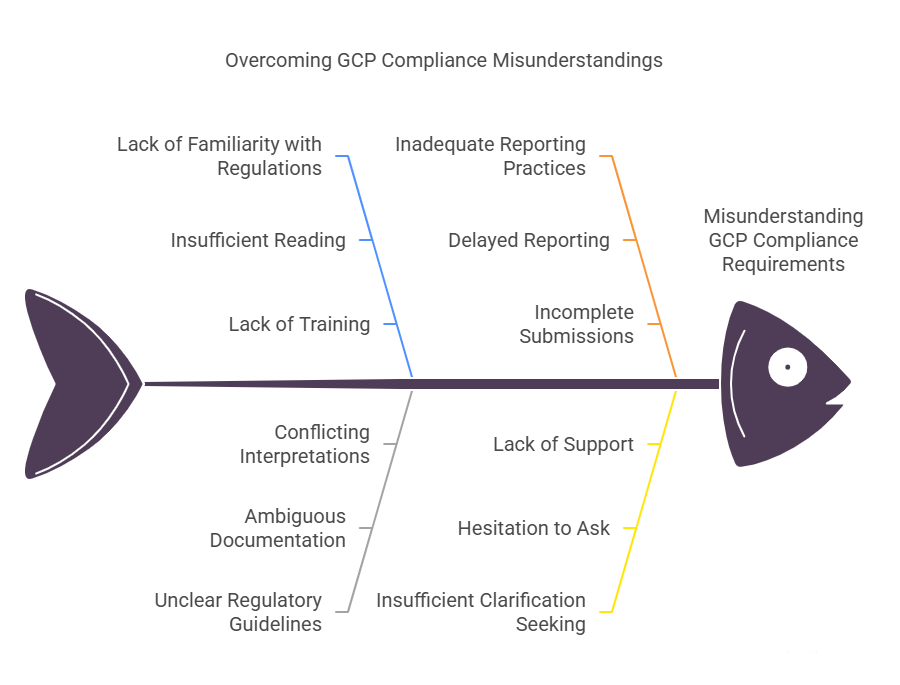
Misunderstanding GCP Compliance Requirements
GCP compliance is a critical part of the clinical trial process, as it ensures that clinical research adheres to ethical and regulatory standards. However, many people misinterpret or misunderstand these compliance requirements, which can lead to mistakes that have serious consequences for the trial and the safety of participants.
What is GCP Compliance?
Compliance means that all involved parties, from researchers to sponsors, follow the established regulations, ensuring that the research is ethically sound and scientifically valid. Common regulatory bodies involved in enforcing GCP include:
FDA (U.S. Food and Drug Administration)
EMA (European Medicines Agency)
WHO (World Health Organization)
These organizations set specific guidelines that must be followed to ensure safety, transparency, and effectiveness in clinical trials. If you don’t fully understand the regulatory requirements, there can be severe consequences, such as trial suspension or invalid data.
Related Blog: How GCP Training Helps Meet Regulatory Requirements
How to Avoid This Mistake:
Read and Familiarize Yourself with Regulations: Spend time understanding the specific compliance requirements set forth by the regulatory bodies relevant to your region or study.
Ask for Clarifications on Specific Regulations: Ensure that you understand the compliance requirements for documentation, participant safety, and data integrity.
Understand Reporting Requirements: Be sure you know how and when to report adverse events, protocol violations, and any other required regulatory submissions.
Failing to Keep Up with GCP Updates
GCP guidelines are updated periodically to reflect changes in medical research practices, technology, and regulatory demands. Failing to keep up with these updates can result in outdated knowledge, which could jeopardize your certification exam or practical application of GCP principles.
Why GCP Updates Matter:
Regulatory Changes: Regulatory bodies frequently update their standards to keep pace with new developments in clinical research. Missing these changes can cause non-compliance and the invalidation of data.
New Technologies and Methods: Advances in research methodologies, clinical trials, and patient data handling mean that previous guidelines may need to be revised or updated.
Emerging Ethical Standards: Ethics in clinical trials is continuously evolving, especially with new technologies like AI and genomics, and these advancements often require updates to GCP principles.
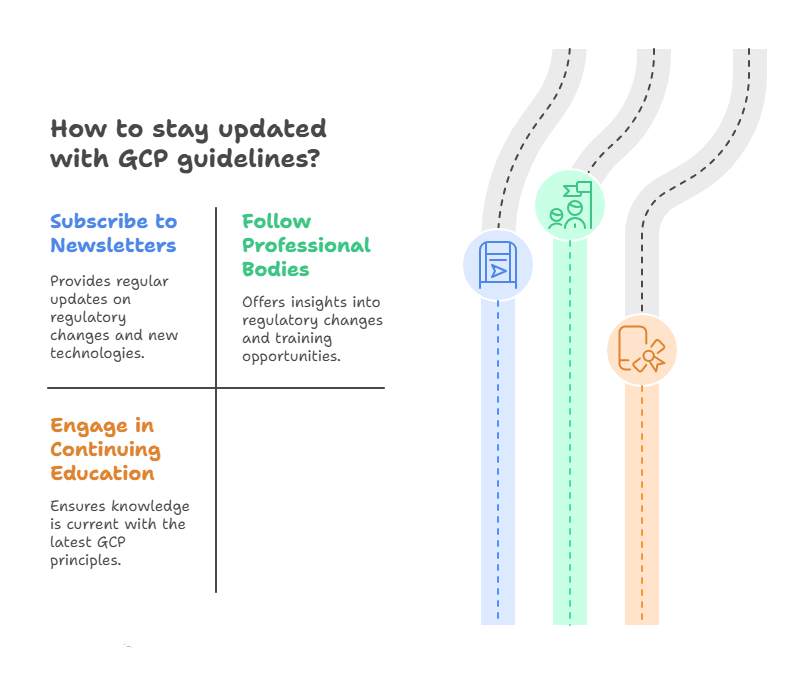
How to Avoid This Mistake:
Subscribe to Industry Newsletters and Journals: Sign up for newsletters and journals related to clinical research and GCP updates. Many institutions and organizations provide periodic updates on GCP.
Follow Professional Bodies and Regulatory Agencies: Join organizations like SoCRA or ACRP, which provide information on regulatory changes and new training opportunities.
Be Proactive in Continuing Education: Participate in refresher courses to keep your knowledge up to date. Take the initiative to learn about any significant changes or updates to the GCP guidelines.
How to Avoid Mistakes During GCP Certification Exams
The GCP certification exam is often the culmination of your training, and many people make mistakes during this exam due to poor preparation or misunderstanding the exam structure. Some common mistakes include time mismanagement, not reading questions thoroughly, or overlooking critical instructions.
Exam-Related Challenges:
Not Familiarizing Yourself with the Exam Format: Without understanding the structure of the exam, you might struggle to allocate time properly or misinterpret question types.
Lack of Practice: Without practicing with mock exams, you may feel overwhelmed by the complexity of the exam, or you may not be used to the types of questions asked.
Rushing Through the Questions: Due to time pressure, you may rush through questions, failing to catch subtle details or missing critical information.
How to Avoid This Mistake:
Understand the Exam Format: Familiarize yourself with the exam's structure—whether it’s multiple-choice, case studies, or scenario-based questions. This will help you prepare accordingly.
Take Practice Exams: Practice exams help you get a feel for the exam’s difficulty and structure, reducing anxiety and helping you improve your time management.
Stay Calm and Read Carefully: Don’t rush. Take time to read each question carefully, ensuring that you fully understand what is being asked before answering.
10 Lesser-Known Facts About GCP:
GCP Has Global Reach: GCP guidelines are implemented internationally to ensure consistency in clinical trials, regardless of location. (Source)
GCP Compliance Reduces Errors: By following GCP guidelines, the chances of making errors in trial execution are reduced significantly. (Source)
GCP Compliance Is Enforced by Audits: Regulatory agencies frequently audit clinical trials to ensure GCP compliance, with serious consequences for violations. (Source)
GCP Training is Part of Many Job Requirements: Many employers in the clinical research industry require GCP certification as part of their hiring process. (Source)
Ethics Committees Are Involved in GCP: Ethical review boards or Institutional Review Boards (IRBs) are key in ensuring GCP compliance during clinical trials. (Source)
GCP Requires Confidentiality: All clinical trial data, including participant information, must be kept confidential according to GCP.
GCP Training Can Lead to Career Growth: Completing GCP training opens doors for career opportunities, including roles as a clinical research coordinator, manager, or monitor.
Risk Management is Critical in GCP: GCP emphasizes risk management, ensuring that trials are conducted with minimal risk to participants.
GCP Is Updated Regularly: Regulatory agencies update GCP guidelines as needed to reflect changes in medical technology and research methods.
Non-Compliance Can Lead to Legal Penalties: Failing to adhere to GCP can result in legal issues, including fines and the invalidation of trial data.
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
By paying attention to these common mistakes during GCP training, you can ensure a smooth and effective learning experience that prepares you for both the certification exams and real-world clinical trials. Avoiding these mistakes will make you a more competent, compliant, and confident clinical researcher.
If you're looking for top-notch GCP training to guide you through the learning process and prepare you for certification, CCRPS is here to help. We offer comprehensive GCP certification programs that will help you advance your career in clinical research.
-
The key principles of GCP include participant safety, data integrity, ethical standards, and compliance with regulatory bodies.
-
You can stay updated by subscribing to industry newsletters, following professional organizations, and attending training sessions and webinars on GCP.
-
The time required depends on the course format, but online courses typically take a few weeks to complete.
-
Yes, GCP training is essential for anyone involved in clinical trials to ensure they comply with ethical and regulatory standards.
-
Yes, many institutions and organizations offer online GCP training programs that are flexible and self-paced.