Difference between ICH GCP and schedule Y
India Form 44 - See below for how trial approval has sped up thanks to 2005 changes by CDSCOSee poster presentation of ICH GCP vs. Schedule Y
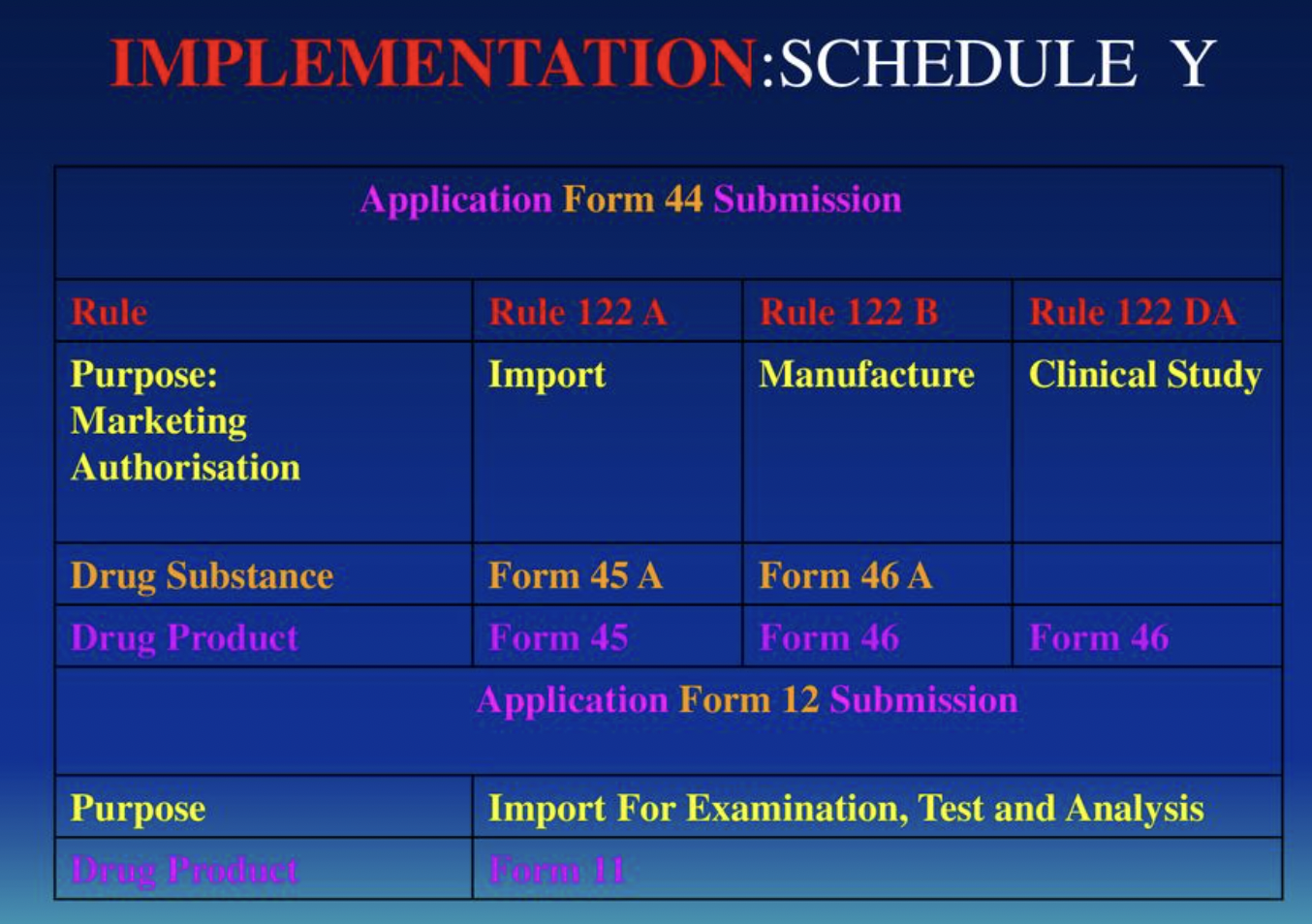
SCHEDULE Y : Download CDSCO Ammendment
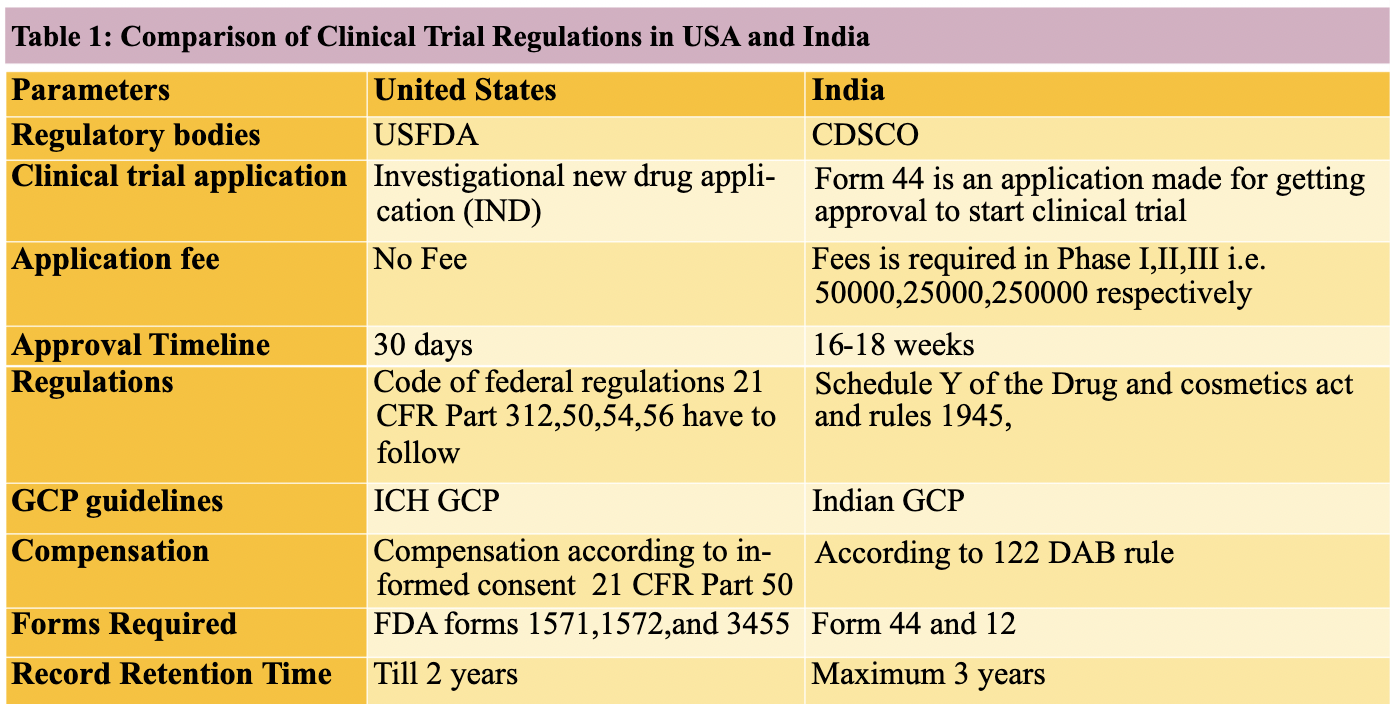
Schedule Y was introduced under the Drugs and Cosmetics Act (1940-1988 -> amended 2005) to introduce requirements for countries to get permission for 1) importing 2) making new drugs 3) conducting clinical trials. Primarily in India due to large patient pool and large biopharma marketshare.
Schedule Y:
1) Depends on status of drug in country of ORIGIN
a) Approved Drugs -> Phase III
b) Not Approved Drugs -> 1 Phase earlier than current phase
c) Newly Discovered Drugs -> Safety data before trial
d) Drug of special relevance -> Trial possible
Potential of clinical research for new therapies -> added Schedule Y to the Drug and Cosmetics Rules of 1945 -> guidelines and requirements for clinical trials.
Schedule Y written with “generics industry in mind” -> lots of trials -> 2005 CDSCO revisions
Definitions for Phase I-IV trials, which eliminated the Phase lag.
Clear responsibilities for investigators; and sponsors.
Requirements for notifying changes in protocol.
Decrease the review time of application from 16 -> 10 weeks
Category A (received Approval in the U.S., Britain, Canada, Germany, South Africa, Switzerland, Australia, Japan and countries in the European Medicines Agency (EMEA)) = 2-4 week approval fast tracking in India
Category B -> 12 weeks
FYI: Nearly all global trials are in the Category A
ICH GCP
Good Clinical Practice (GCP) is an international standard set for conducting, inventing, recording, and reporting clinical trials which may involve humans as participants. It's very important to obey this standard as it offers the public the confidence that the trial subjects' rights, safety, and well-being are protected, and that data in clinical trials are plausible.
The target of the ICH GCP (International Conference on Harmonization of Good Clinical Practice) is to offer a uniform standard for U.S., European Union, and Japan to facilitate the adoption of clinical data by regulatory authorities of the stated jurisdictions. The guidelines must be followed when information from clinical trials must be submitted to regulatory authorities.
The Indian version of GCP relies on the ICH-GCP, however there are crucial differences between the two. A few of the guidelines utilized at the Indian variant lead to the tricky methodology that becomes overwhelming for sponsors and investigators.
Difference between ICH GCP and schedule Y
The Indian principles state that the copy for the SOPs have to be signed by both the investigator and the sponsor.
The investigator, together with his research team, should comply with the SOPs. This could not be possible as it will grow to be a huge burden for patrons to get the SOPs signed with all the investigators of this trial. The full procedure of keeping several SOPs and making alterations are complicated enough.
The role of the investigator in data evaluation, as stated by the ICH-GCP, will be to submit a recap of this trial and its outcomes to the Sponsor and its Ethics Committee, although the Indian GCP mentions that the investigator or the institution ought to analyze the data, create a study report, and submit it to the Sponsor and Ethics Committee. Additionally, this will result in various study reports for various sites of a similar study.
ICH GCP vs Schedule Y
Foundations of Clinical Trial Regulations
ICH GCP and Schedule Y serve as benchmarks in clinical research, setting the standards for trial conduct, participant safety, and data integrity. Here, we explore their origins, objectives, and the roles they play in the global and Indian contexts, respectively.
History and Evolution of ICH GCP
Delve into the development of ICH GCP guidelines, a result of collaborative efforts between the United States, the European Union, and Japan. Understand the critical updates that have shaped these guidelines to address contemporary challenges in clinical research.
The Genesis and Revisions of Schedule Y
Trace the evolution of Schedule Y from its initial introduction under India’s Drugs and Cosmetics Act to its significant amendments, reflecting India’s growing prominence in the global pharmaceutical landscape.
Scope and Application: Global vs. National
Examine the global reach of ICH GCP guidelines versus the specific application of Schedule Y in India. This section highlights how each framework addresses the scope of trials from multinational to local contexts.
Approval Processes: Bridging International and Local Needs
Unpack the differences in approval processes under both frameworks, emphasizing Schedule Y’s approach to fast-tracking drugs already approved in developed nations and how it contrasts with ICH GCP’s emphasis on uniformity across diverse regulatory environments.
Investigator Responsibilities
Contrast the detailed responsibilities of investigators under both frameworks, with a focus on the additional requirements placed on Indian researchers by Schedule Y.
Pharmacovigilance Under Schedule Y and ICH GCP
Explore the stringent drug safety monitoring guidelines set by ICH GCP and how they compare with the detailed post-approval safety requirements under Schedule Y. This section provides insights into effective pharmacovigilance practices tailored to both global and local contexts.
Managing Protocol Changes
Discuss the protocols for managing changes in clinical trials, comparing the mandatory notifications required by Schedule Y with the more flexible regional submissions endorsed by ICH GCP.
Schedule Y in Indian Jurisprudence and Pharmacy
Analyze the legal framework underpinning Schedule Y, emphasizing its role in shaping ethical drug trials in India. This chapter also discusses how adherence to Schedule Y impacts pharmaceutical practices and trial structures.
Ethical Considerations in Global and National Trials
Address the ethical standards upheld by ICH GCP and how these integrate with local regulations like Schedule Y to protect trial participants and ensure data reliability.
Harmonizing ICH GCP with Schedule Y
Explore potential pathways for integrating ICH GCP guidelines more closely with Schedule Y, aiming to streamline approval processes while enhancing patient safety and data integrity.
Predictions for Global and Indian Clinical Trial Landscapes
Forecast the future of clinical trials both globally and in India, considering technological advancements, regulatory changes, and the increasing importance of ethical and safety standards.
FAQs
What is the difference between GCP and ICH?
Good Clinical Practice (GCP) is a set of internationally recognized ethical and scientific quality requirements that must be observed for designing, conducting, recording, and reporting trials that involve the participation of human subjects. The International Conference on Harmonization (ICH) GCP guidelines are a specific set of standards within GCP, established to ensure a uniform standard across the US, EU, and Japan to facilitate mutual acceptance of clinical data by the regulatory authorities in these jurisdictions.
What is Schedule Y in clinical research?
Schedule Y is a part of the Indian Drugs and Cosmetics Act that provides guidelines for conducting clinical trials in India. It outlines the requirements for permission to import and/or manufacture new drugs for sale or to undertake clinical trials in India. Schedule Y specifies the necessary data to be submitted, the procedures for obtaining permissions, and the requirements for conducting clinical trials in terms of safety and efficacy of the drug.
What is the ICH GCP?
The ICH GCP (International Conference on Harmonization of Good Clinical Practice) is a set of standards for clinical trials that provides a unified standard protocol for the EU, Japan, and the US to facilitate the mutual acceptance of clinical data by the regulatory authorities in these jurisdictions. It aims to protect the rights, safety, and well-being of trial participants while ensuring the integrity of clinical trial data.
How do Schedule Y and ICH GCP differ in their approach to trial approvals?
Schedule Y allows for a fast-track approval process for drugs that have already been approved in certain major international markets (U.S., UK, Canada, Australia, and Japan), whereas ICH GCP requires uniformity in the conduct of trials and does not differentiate based on the drug's approval status in different countries.
Can international clinical trial data be directly used for drug approval in India under Schedule Y?
Yes, international clinical trial data can be used for drug approval in India under Schedule Y, provided that the data meets the requirements set forth by the Indian regulatory authorities. This includes ensuring that the trials are conducted in accordance with GCP and that the data is relevant and applicable to the Indian population.
What are the specific responsibilities of investigators under ICH GCP compared to Schedule Y?
Under ICH GCP, investigators are responsible for ensuring the rights, safety, and welfare of participants are protected, ensuring that the clinical trial data are accurate, and conducting the study in compliance with the approved protocol. Schedule Y also emphasizes similar responsibilities but includes additional requirements such as mandatory submission of periodic safety update reports and final study reports to the Indian regulatory authorities.