How GCP Training Enhances Data Accuracy in Clinical Trials
Good Clinical Practice (GCP) training is essential in clinical trials to ensure that data collected is accurate, reliable, and trustworthy. GCP provides the guidelines and standards for designing, conducting, and monitoring clinical trials while safeguarding participants' rights and well-being. One of the critical areas where GCP plays a pivotal role is in ensuring data accuracy throughout the trial process. Accurate data is essential for drawing valid conclusions and for the trial’s success. This blog will cover the importance of accurate data collection, the role of GCP training in preventing data errors, and techniques used to maintain high data integrity.
Importance of Accurate Data Collection and Reporting
Accurate data collection and reporting form the backbone of any clinical trial, as they directly impact the reliability of the results. Here’s why it is crucial:
Validity of Results: Inaccurate data can lead to false conclusions, undermining the entire trial’s purpose. Clinical trials rely on precise measurements, assessments, and reports to determine the effectiveness and safety of treatments.
Regulatory Compliance: Regulatory authorities, such as the FDA or EMA, require clinical trials to follow stringent data accuracy guidelines. Failure to report data accurately can result in legal consequences, trial suspension, or invalidation of results.
Patient Safety: Accurate data collection is not only necessary for research outcomes but also for monitoring patient safety. Ensuring that all health parameters and adverse events are reported correctly is vital for participant protection.
GCP training emphasizes that data collection should be thorough, consistent, and precise to ensure valid study results and participant safety. Without it, the integrity of the clinical trial is compromised, and the research community’s trust in the findings diminishes.
Related Blog: GCP Certification: What You Need to Know
Data Integrity Checks Covered in GCP Training
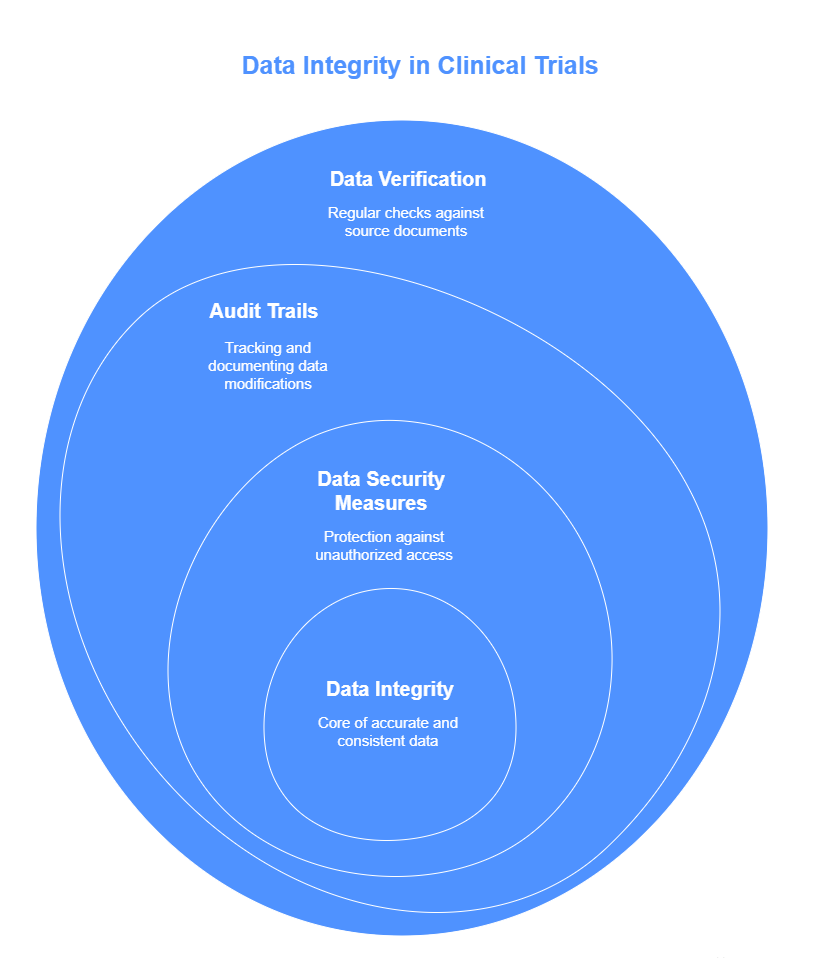
Data integrity refers to the accuracy and consistency of data over its lifecycle. GCP training equips research teams with the necessary knowledge and tools to ensure data integrity throughout the clinical trial process. This involves:
Data Verification: GCP training teaches the importance of regularly verifying the accuracy of collected data against source documents. This includes cross-checking patient records, lab results, and other clinical measurements to confirm they match the data recorded in trial databases.
Audit Trails: One of the key elements of GCP training is understanding audit trails—systems that track and record every modification made to the data. These systems ensure that any changes or updates to the data are documented, providing transparency and accountability.
Data Security Measures: Training also covers the security of patient and study data. GCP standards dictate how data should be stored, protected from unauthorized access, and backed up to prevent data loss, ensuring its integrity throughout the study.
With proper data integrity checks, clinical trial teams can ensure that the data is trustworthy, accurate, and compliant with regulatory standards, mitigating the risks of data discrepancies.
Related Blog: The GCP Training Process: Step-by-Step Guide
How GCP Helps Mitigate Data Entry Errors
Data entry errors are a common challenge in clinical trials. GCP training addresses this issue by providing the knowledge and tools to minimize such errors:
Standardized Data Entry Protocols: GCP emphasizes the use of standard operating procedures (SOPs) for data entry. SOPs ensure that all data is entered in a consistent format, reducing the risk of mistakes during transcription or input into trial systems.
Training on Data Management Systems: Research teams are trained on using data management systems that are designed to reduce human errors. These systems often come with built-in validation checks, prompting users to correct inconsistencies or missing information before submission.
Monitoring and Double-Checking: GCP highlights the importance of regularly monitoring and double-checking data entered into trial databases. A second review or verification step helps catch errors that may have been missed during the initial entry.
By emphasizing data entry accuracy, GCP training helps prevent small mistakes from becoming large issues that could potentially jeopardize the trial’s validity.
Techniques for Ensuring Data Accuracy in Clinical Trials
Several techniques are taught in GCP training to ensure that data remains accurate throughout the clinical trial process. These include:
Source Data Verification (SDV): One of the most common techniques used in clinical trials is source data verification, where data is cross-checked against the original source documents (e.g., patient records, lab results). This ensures that what is recorded in the trial database matches the original information.
Consistent Data Collection: GCP training teaches research teams how to collect data consistently by standardizing protocols for patient visits, assessments, and measurements. This minimizes the chances of inconsistent data entry due to variation in how data is collected across different sites or by different researchers.
Centralized Data Management: To avoid errors that may arise from decentralized data collection, GCP training encourages the use of centralized data management systems. These systems consolidate data from multiple sites, ensuring consistency and providing real-time monitoring to detect any discrepancies early in the process.
By following these techniques, clinical trials can maintain a high level of data accuracy, allowing researchers to draw reliable conclusions and meet regulatory standards.
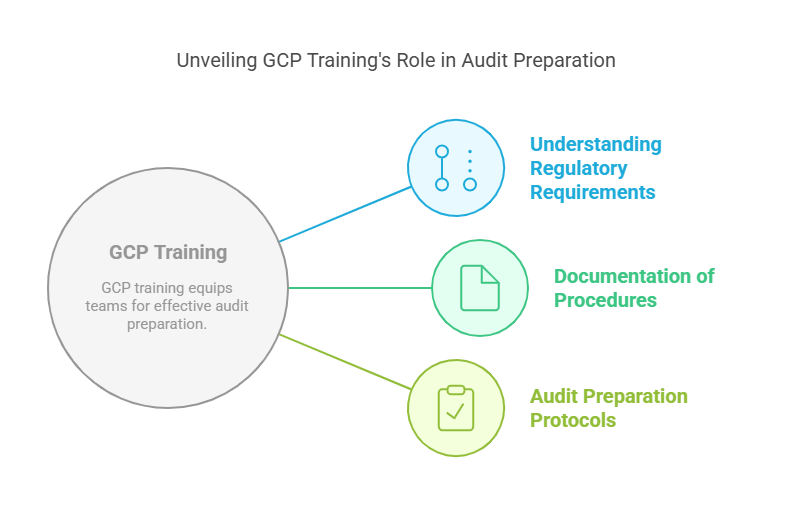
How GCP Training Helps in Audit Preparation
Audit preparation is an essential part of clinical trial management. GCP training provides critical knowledge to help trial teams prepare for both internal and external audits. Here's how GCP training aids in the audit process:
Understanding Regulatory Requirements: GCP training ensures that research staff are aware of all regulatory requirements for documentation, reporting, and data handling. This knowledge helps them prepare the necessary materials for audits and ensures that they can demonstrate compliance with GCP standards.
Documentation of Procedures: During a clinical trial, all actions, from patient recruitment to data collection and analysis, must be thoroughly documented. GCP training reinforces the importance of maintaining detailed and accurate records that will be needed during an audit.
Audit Preparation Protocols: GCP training provides researchers with protocols for audit preparation, including how to organize data, ensure audit trails are intact, and confirm that all required documents are available. Proper preparation minimizes the risk of audit findings and ensures that trials can withstand regulatory scrutiny.
Being well-prepared for audits ensures that any trial data can be scrutinized and verified against the original documentation, maintaining the trial’s credibility and compliance with ethical standards.
10 Lesser-Known Facts About GCP
GCP applies to all clinical trials involving human participants, including those for medical devices, vaccines, and behavioral studies. (Source)
Data management systems used in clinical trials are often equipped with built-in checks to flag inconsistencies or missing data during the entry process. (Source)
Audit trails in GCP not only track changes but also record who made them, ensuring accountability and transparency. (Source)
Clinical trial data must be stored securely for a minimum period, often several years, to comply with regulatory requirements. (Source)
The FDA mandates GCP adherence for all clinical trials it oversees, which is a key factor in the approval of new drugs and therapies. (Source)
Data errors often arise from transcription mistakes or inconsistent data entry methods, which GCP aims to minimize.
Real-time monitoring of data is possible in centralized data management systems, helping to identify errors early.
Internal audits are conducted regularly to ensure that GCP standards are being maintained throughout the trial process.
Randomization techniques under GCP reduce biases in data collection and ensure more accurate and generalizable results.
GCP includes specific provisions for dealing with adverse events and unanticipated problems during a clinical trial, ensuring patient safety and data integrity.
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
GCP training plays an indispensable role in ensuring that clinical trials produce accurate, reliable, and trustworthy data. From data integrity checks to audit preparation, GCP training equips researchers with the necessary tools and techniques to ensure data accuracy throughout the trial process. By minimizing errors, standardizing data collection, and ensuring continuous monitoring, GCP safeguards the integrity of clinical trial results, leading to valid conclusions that improve patient care and advance medical science.
In conclusion, adherence to GCP standards, bolstered by comprehensive training, is critical to enhancing data accuracy in clinical trials. By mitigating errors, standardizing procedures, and preparing for audits, clinical trials can maintain the highest levels of data integrity. At CCRPS, we provide thorough GCP training to ensure your clinical trials are compliant and data-driven.
-
GCP is a set of guidelines that ensure clinical trials are conducted with ethical standards, ensuring the safety of participants and the accuracy of data collected.
-
Accurate data is crucial for making reliable decisions about the safety and effectiveness of new treatments. It ensures the integrity of the trial and the trustworthiness of the results.
-
GCP training teaches standardized procedures, the use of data management systems, and verification techniques to reduce the likelihood of errors in data entry.
-
SDV is a process where trial data is checked against original source documents to confirm its accuracy and reliability.
-
GCP training helps researchers understand regulatory requirements, maintain accurate documentation, and prepare the necessary materials for internal and external audits.