How To Get a Pharmacovigilance Certification in Canada: Everything You Need to Know in 2025-2026
In 2025, pharmacovigilance certification isn’t optional if you want to work in Canada’s drug safety, biotech, or regulatory affairs ecosystem. Whether you're targeting roles at Health Canada, major CROs like ICON and Syneos, or pharma firms like Apotex and Bausch Health, certification is the minimum bar for serious consideration. Entry-level safety associate roles without certification cap at around CAD $52,000/year, while certified PV specialists with applied knowledge and audit readiness regularly earn CAD $80,000–$98,000—a 60% salary delta purely based on qualifications, not years of experience.
This isn't just about learning ICH-E2E or MedDRA coding—it’s about qualifying for roles that influence regulatory submissions, risk management plans, and international pharmacovigilance agreements (IPVAs). Without a credentialed course that aligns with global expectations, Canadian employers won’t sponsor work permits, contract upgrades, or lead roles. Certification is your leverage—for remote work eligibility, global transfers (EMA, TGA, FDA-compliant roles), and internal mobility within Canada’s GVP workforce.
What Is Pharmacovigilance Certification in Canada Exactly? Skills Required and Jobs Explained
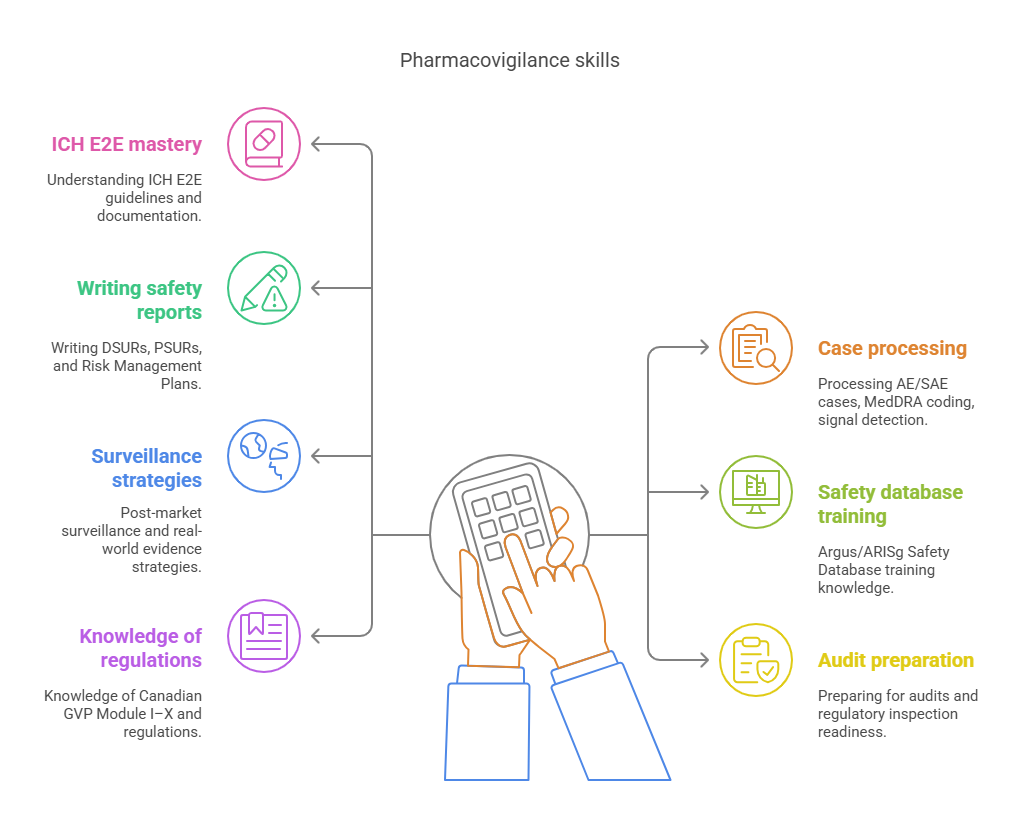
Pharmacovigilance certification in Canada refers to structured, regulatory-aligned training that prepares professionals to monitor, assess, and report adverse drug events (ADEs) in line with Health Canada, ICH-E2E, and WHO UMC standards. It is not generic clinical research training—it’s specialized education in pre- and post-marketing safety surveillance, risk minimization strategies, and GVP compliance (Good Pharmacovigilance Practices).
Employers don’t just look for knowledge—they assess if you can operate inside global safety databases (like ARISg or Argus), write PSURs and RMPs, and support regulatory inspections. These skills directly tie to job titles like Drug Safety Associate, PV Scientist, and Regulatory Affairs Officer—roles that are in short supply across Ontario, British Columbia, and Quebec due to growth in biotech and post-COVID regulatory expansion.
Why Should You Get Pharmacovigilance Certification to Work in Canada?
In Canada’s competitive life sciences ecosystem, pharmacovigilance roles are now regulated by demonstrated capability, not just education. Health Canada, CROs, and sponsor companies prioritize certified professionals due to growing pressure for regulatory compliance, international harmonization (ICH, WHO), and risk traceability in safety data. If you're uncertified, you remain locked out of global submissions, PV inspections, and leadership in safety signal review boards. A certified professional, however, gets fast-tracked into auditable roles, remote global opportunities, and higher pay brackets. In provinces like Ontario and British Columbia, recruiters will not even shortlist non-certified candidates for pharmacovigilance or drug safety roles above the assistant level.
| Factor | With Pharmacovigilance Certification | Without Certification |
|---|---|---|
| Job Title Access | Drug Safety Associate, PV Officer, Regulatory Affairs Executive | Data Entry Assistant, Intern, Documentation Support |
| Hiring Consideration | Shortlisted for Health Canada-aligned and EMA-linked roles | Ignored for mid-to-senior roles in CROs and sponsors |
| Salary Range (CAD) | $78,000 – $102,000 | $42,000 – $58,000 |
| Remote/Global Work Eligibility | Eligible for EU, UK, and US sponsor audits and global submissions | Restricted to local, manual PV documentation tasks |
| Promotion Timeline | 12–18 months into Manager or Auditor roles | Stagnates for 3–5 years before basic promotion |
| Regulatory Audit Involvement | Qualified to lead internal audits and respond to inspections | Excluded from inspection-facing or sponsor-facing work |
Which Certification Should You Choose to Become a PV Professional in Canada?
Pharmacovigilance certification options in Canada range from open-access webinars to limited-scope university diplomas. While platforms like DIA or Coursera offer theoretical overviews, and some institutes bundle PV into broader clinical research courses, most lack compliance depth, inspection readiness, and real-case simulation. They often skip over RMPs, fail to prepare for DSUR/PSUR authorship, and don’t provide Health Canada-specific audit SOPs. That leaves learners with a credential—but no readiness to step into GVP-facing roles.
The Certified Clinical Research Professionals Society (CCRPS) offers a CPD-accredited Advanced Pharmacovigilance & Drug Safety Certification that fills this gap. Designed specifically for global PV careers with Canadian compliance in mind, it includes 70+ scenario-driven modules, case processing via Argus simulations, and practical deliverables (AE classification charts, risk templates, CAPA plans). Unlike generic platforms, CCRPS offers both self-paced and bootcamp formats, access to live instructors with inspection experience, and transparent bios of the curriculum creators. This is built not by marketers or faceless teams—but by regulatory professionals who’ve sat across Health Canada inspectors.
| Comparison Basis | Other Certification Providers | CCRPS Pharmacovigilance Certification |
|---|---|---|
| Accreditation | No formal CPD/CME or audit-aligned credentials | CPD-accredited; Health Canada, EMA, FDA audit-prep aligned |
| Curriculum Depth | Introductory, theory-based, lacks real PV documentation | 70+ modules; includes AE case reports, PSURs, DSURs, and RMPs |
| Learning Format | Static self-paced only | Self-paced + optional live bootcamp with regulatory simulations |
| Instructor Access | No direct contact; anonymous video content | Live 1-on-1 coaching from experienced PV scientists and GVP auditors |
| Team Transparency | No curriculum team listed publicly | CCRPS lists instructors, credentials, and contact profiles |
| Flexibility & Support | No installment plans or future-proofing | Interest-free payments, lifetime access, refresher updates every 2 years |
Why CCRPS’s Pharmacovigilance Certification Will Be a Game Changer for Your Career in Canada
In Canada, the pharmacovigilance job market is increasingly salary-banded based on compliance-readiness and credentialing. Employers like Roche Canada, Bayer, and GSK routinely offer tiered compensation models—where certified professionals start at higher pay grades with faster promotion timelines. In provinces like Ontario and British Columbia, the salary difference between a certified PV associate and a non-certified one exceeds CAD $30,000 annually. More importantly, certified candidates qualify for hybrid/remote global PV roles, which offer even greater pay flexibility and bonuses.
Below is a data-backed comparison of salary outcomes before and after obtaining CCRPS’s certification, based on 2024–2025 recruiter and job market intelligence from Canada’s top three hiring regions: Ontario, Quebec, and Alberta.
Summarizing All You Need to Know About Getting Your Pharmacovigilance Certification in Canada
If you're aiming to build a career in pharmacovigilance within Canada’s life sciences sector, certification isn't optional—it’s strategic leverage. Without it, you remain stuck in low-skill roles with zero regulatory impact and no path to cross-border or hybrid PV work. With a certification—especially one as comprehensive as CCRPS’s CPD-accredited program—you not only gain practical, regulatory-aligned expertise but also command higher salaries, better job mobility, and long-term audit-readiness that most employers actively seek.
| Key Factor | Details |
|---|---|
| Is Certification Mandatory? | Yes, for roles in CROs, pharma companies, and Health Canada-aligned positions |
| Recommended Provider | CCRPS – Certified Clinical Research Professionals Society |
| Accreditation | CPD-certified, aligned with ICH-E2E, GVP Modules I–X, and global PV standards |
| Curriculum Features | 70+ modules, AE/SAE case studies, PSURs, DSURs, audit prep, CAPA templates |
| Learning Modes | Self-paced + Live Bootcamp (optional) |
| Instructor Access | Live coaching available from PV scientists and GVP audit experts |
| Expected Salary Increase | CAD $25,000–$60,000+ depending on role and province |
| Ideal For | Graduates, PV associates, clinical researchers, and QA professionals entering pharmacovigilance |
| Payment & Support | Interest-free plans, lifetime access, refresher updates every 2 years |
Frequently Asked Questions
-
Not realistically. Most employers in Canada—especially in provinces like Ontario, Alberta, and British Columbia—require job-ready, audit-prepared candidates for pharmacovigilance roles. A general science or pharmacy degree might qualify you for internships or document assistant roles, but you won’t be eligible for safety associate or regulatory-facing positions without formal certification. Health Canada-compliant roles often demand ICH-GVP alignment, AE/SAE case processing proficiency, and audit traceability—none of which are taught in traditional degrees. That’s where certification, especially from a global provider like CCRPS, becomes critical for practical entry and salary leverage.
-
CCRPS’s Advanced Pharmacovigilance & Drug Safety Certification is fully self-paced, allowing completion within 4 to 12 weeks, depending on your schedule. The average learner working part-time finishes it in 6–8 weeks, including assignments, quizzes, and project simulations. If you opt for the bootcamp version, you’ll complete weekly live sessions and compliance drills over 4–6 weeks. Unlike static programs, CCRPS also gives lifetime access, meaning you’ll receive updated modules every 2 years at no extra cost—essential for staying compliant with Health Canada and EMA audit expectations.
-
Yes—especially if the certification includes GVP Module training, DSUR/PSUR authorship practice, and Argus/ARISg exposure like CCRPS offers. Recruiters for EMA, TGA, and FDA-facing pharmacovigilance teams prioritize candidates with compliance-ready certification over those with generic diplomas. CCRPS’s program aligns with ICH-E2E and includes real-world templates for RMPs, CAPA plans, and audit readiness, which positions you for remote or hybrid roles with global CROs and sponsors operating out of Canada or hiring Canadian-regulated staff.
-
With CCRPS certification, typical post-completion salaries in Canada range from CAD $78,000 to $105,000, depending on role and location. For example, certified PV associates in Ontario start around CAD $82,000, while documentation specialists with PSUR/DSUR responsibilities can earn up to CAD $98,000. Without certification, even highly educated professionals remain capped at CAD $50,000–$60,000 in assistant-level roles. Beyond base pay, certified candidates also receive better bonus structures, global project exposure, and faster promotion timelines.
-
Yes. CCRPS is internationally recognized and CPD-accredited, which holds weight across Canadian life sciences recruiters, CROs, and sponsor pharma firms. Their curriculum maps directly to ICH E2E, GVP Modules, and Health Canada’s compliance frameworks. In 2024–2025, CCRPS-certified professionals were hired by teams at Apotex, Bausch Health, IQVIA Canada, and Canadian branches of Roche and Pfizer. What sets CCRPS apart is that recruiters actually understand its audit-ready design, making it more than just a certificate—it’s an employability asset.