Medical Research Jobs

The landscape of clinical research is constantly evolving, offering exciting opportunities for individuals passionate about improving human health. Here's a glimpse into some key roles in this fulfilling field (salary ranges are estimates based on 2024 data from bls.gov :
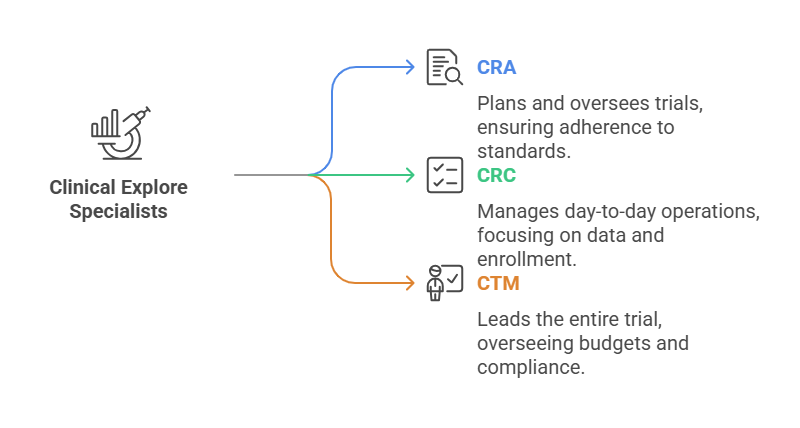
Clinical Explore Specialists:
Clinical Examine Relate (CRA): Plans and oversees clinical trials, ensuring adherence to traditions and reporting comes almost (Emolument: $50,000 - $90,000) Consider CCRPS CRA Certification.
Clinical Ask around Facilitator (CRC): Manages the day-to-day operations of clinical trials, checking data collection, part enrollment, and tradition checking (Stipend: $43,000 - $55,000). Look for after CCRP Certification.
Clinical Trials Executive (CTM): Leads the entire clinical trial plan, overseeing budgets, timelines, and authoritative compliance (Remuneration: $70,000 - $90,000) See into CCRPS CCTM Certification.
Lab and Data Examination Experts:
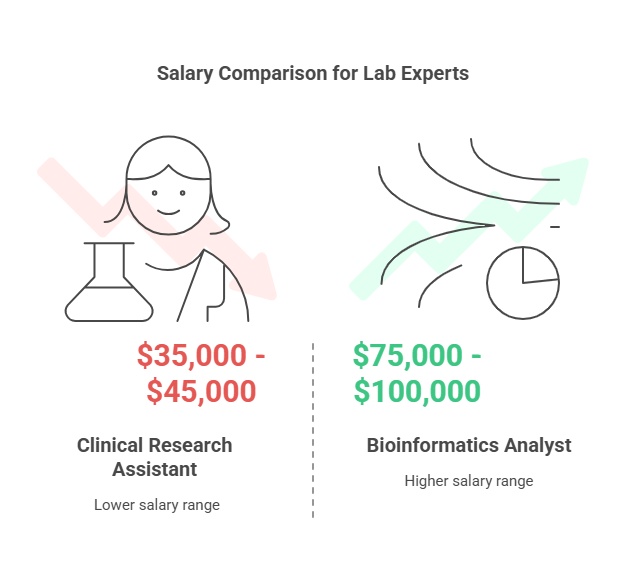
Clinical Research Assistant (CRA): Performs laboratory tests following established protocols to support research studies (Salary: $35,000 - $45,000). Consider CLT Certification through CCRPS.
Bioinformatics Analyst: Leverages computer tools and biological data to analyze complex data from clinical research labs (Salary: $75,000 - $100,000). Review CCRPS CBS Certification.
Communication and Regulatory Professionals:
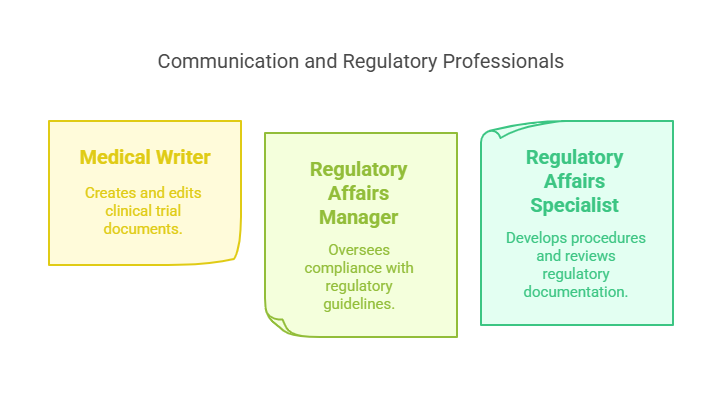
Medical Writer: Creates and edits documents for clinical trials, including protocols and reports for dissemination (Salary: $60,000 - $80,000) Target CCRPS CMW Certification.
Regulatory Affairs Manager: Oversees the regulatory process, ensuring clinical research complies with guidelines (Salary: $95,000 - $125,000) Consider CCRPS CARM Certification.
Regulatory Affairs Specialist: Develops procedures for clinical trial applications, reviews protocols, and maintains regulatory documentation (Salary: $55,000 - $100,000) Explore Pharmacovigilance Certification through CCRPS.
Clinical research and career opportunities :
1. CRA Certification (Clinical Research Associate):
Clinical Research Associate (CRA): Plans and oversees clinical trials, ensuring adherence to regulations and accurate reporting (Salary: $50,000 - $90,000). Consider obtaining [CCRPS CRA Certification](https://app.ccrps.org/courses/cra) to advance your expertise in clinical trial management.
2. CRC Certification (Clinical Research Coordinator):
Clinical Research Coordinator (CRC): Manages day-to-day operations of clinical trials, including data collection and participant enrollment (Salary: $43,000 - $55,000). Enhance your capabilities with CCRP Certification ( https://app.ccrps.org/courses/Clinical-Research-Coordinator ) tailored for coordinators.
3. Clinical Trials Manager (CTM):
Leads the entire clinical trial program, overseeing budgets, timelines, and regulatory compliance (Salary: $70,000 - $90,000). Explore CCRPS for comprehensive management skills.
4. Clinical Research Assistant:
Performs laboratory tests to support research studies (Salary: $35,000 - $45,000). Consider enhancing your laboratory skills with CCRPS
5. Bioinformatics Analyst:
Leverages computer tools to analyze biological data from clinical research labs (Salary: $75,000 - $100,000). Improve your data analysis capabilities with CCRPS CBS Certification.
6. Medical Writer:
Creates and edits clinical trial documents (Salary: $60,000 - $80,000). Target your writing skills with CCRPS CMW Certification
7. Regulatory Affairs Manager:
Ensures clinical research compliance with guidelines (Salary: $95,000 - $125,000).
8. Regulatory Affairs Specialist: Develops procedures for clinical trial applications and maintains regulatory documentation (Salary: $55,000 - $100,000).
Explore Courses for Clinical Research Career
Courses Available:
