The Ultimate Guide to Becoming a Clinical Research Associate
The ultimate guide to becoming a clinical research associate by CCRPS
A Clinical Research Associate (CRA) is a critical role in the implementation of clinical trials to develop new medical therapies. They guarantee the proper conduct of studies through proper ethics, following the laws and recording data correctly. CRA's assist in the trial surveillance from the beginning to the end, interfacing mostly with researchers, doctors and sponsors to guarantee that the process is going as planned.
As new medicines and treatments come into the market there is a rising need for clinical research associates. Many companies, including pharmaceutical firms and hospitals, are looking for skilled professionals to oversee clinical trials. It provides strong job security, competitive pay, and plenty of growth opportunities.
To succeed as a clinical research associate, you must understand clinical trial protocols, regulatory requirements, and CRA training programs. It is also useful to have knowledge about some important tools, such as the EU clinical trials registry and GCP monitoring. This will put you in a position to advantage in this field to get the right education and experience.
What Does A Clinical Research Associate (CRA) Do?
A Clinical Research Associate (CRA) is there to oversee clinical trials to make sure they are “morally right” as well as “physically safe” and are conducted according to the set research protocols. They work with doctors, researchers, and sponsors to achieve a successful trial. CRAs contribute to the safety of patients by observing the patients, data collected, and the study’s conduct of the study in relation to the guidelines laid down by the government. They can include reviewing trial sites, reports, and solving any problems that may occur during the research. They are therefore very important in the generation of new treatments while at the same time upholding high standards of quality and compliance.
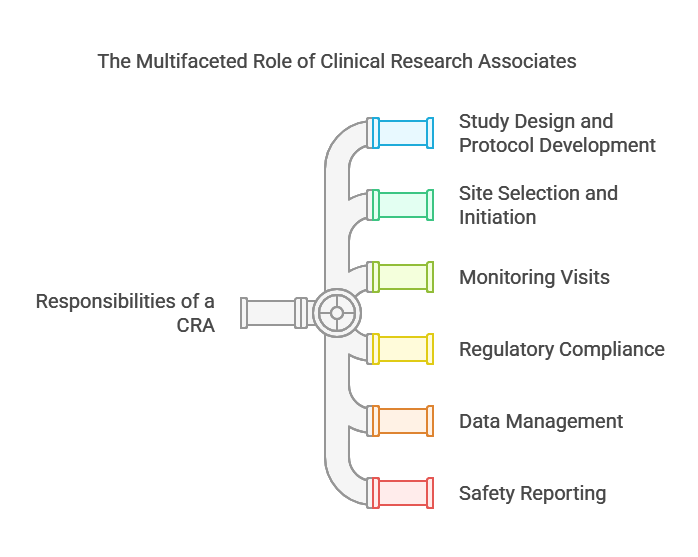
Key Responsibilities Of A CRA:
Clinical trials must be closely monitored to make sure they adhere to the right procedures and that the data collected is accurate. One of the roles that I will be performing during this process is that of a Clinical Research Associate (CRA), who assists in the management of the studies and guarantees that they are executed as they should. They are responsible for helping to ensure the protection of human subjects and the integrity of the scientific data, which in turn supports the development of safer and more effective treatments. To better understand their role, please allow me to elaborate on the primary duties encountered in clinical research.
Study Design And Protocol Development
A Clinical Research Associate (CRA) is an important worker in the execution of the study as they assist in the formulation of the study protocols. Study protocols are detailed instructions of how a clinical trial is to be conducted and CRAs ensure that the protocols they develop are scientifically sound and meet the necessary regulations. Correctly formulated protocol will help to avoid mistakes, ensure the safety of patients and provide order during the trial. As a result, reliable data can be produced that can help in the development of new treatments and therapies. CRA also plays a role in the assessment of these protocols to ensure that the study is conducted without difficulties at one point to another.
Site Selection And Initiation
It is an important step to select appropriate location for the clinical trial. In order to ensure that the site is suitable for the study, a Clinical Research Associate (CRA) assesses the facilities/infrastructure, equipment and training of the staff. When the site is identified, the CRAs helps in the start up process by educating the site personnel on the study and the procedures to be followed in the trial. This training is very important as it ensures that everyone who is involved in the process knows what is expected of them and how to go about it in the right way to avoid making any mistakes. In this way, CRAs ensure that the trial is conducted without any problems and that the results are accurate and credible by ensuring that the sites are properly chosen and prepared to receive the participants and that the whole process is done in a safe and legal way.
Monitoring Visits
These visits are important to ensure the quality and accuracy of the clinical trials on a regular basis. These visits ensure that the study is carried out according to the approved study protocols and the regulatory guidelines. A CRA reviews patient documents, compares the information in the source documents with that in the CRFs, and checks for the completeness and accuracy of the entered data. They also sit through site staff to ensure that the right processes are being followed and, where necessary, resolve any problems that may come up. These visits are performed by the CRAs in order to guarantee the data integrity, patient safety and to achieve accurate results of the study.
Regulatory Compliance
Adherence to strict regulatory compliance is mandatory in clinical research in order to protect patients and produce accurate results. Clinical trials have to fulfill both national and international standards, among them Good Clinical Practice (GCP). A Clinical Research Associate (CRA) monitors whether all trial procedures are in accordance with these standards through documentation, procedures, and ethical requirements. They are aware of the changes in the regulations and engage in the research teams to avoid compliance failures. Through achieving the highest standards throughout the study, the CRAs offer protection to the participants and the credibility of the research results.
Data Management
Accurate and properly organized data is important for the success of a clinical trial. A Clinical Research Associate (CRA) makes sure that all data is being gathered properly, checked and documented in the right manner as per the study protocol. They go through the CRFs and other paperwork to check for any errors or omissions and address them. If there are any errors or inconsistencies in the data, the CRAs report them to site staff for correction. They also make sure that the data is entered in the right manner, in compliance with the regulatory standards to avoid mistakes that might affect the results of the study. In this manner, CRAs guarantee that the data collected is of high quality and, therefore, supports the credibility of the trial and its findings in subsequent medical advancements.
Safety Reporting
The safety of participants is of great concern in clinical trials and a Clinical Research Associate (CRA) is involved in the process of monitoring and reporting of any ill health that may be incurred during the study. In case of an adverse event (AE) or serious adverse event (SAE), the CRA ensures that it is documented and reported to the right channels. They implement pharmacovigilance in the identification, evaluation and control of the risks that are associated with the trial. Through proper handling of safety reports and making sure that the reports are in compliance with the set rules and regulations, CRAs ensure that ethical research is well maintained and participants’ safety is protected.
Communication
Effective communication in clinical trials is essential. A Clinical Research Associate (CRA) serves as a connection between the sponsor, clinical sites, and regulatory authorities to keep everyone informed of the trial's progress. They carry important information, respond to questions, and assist in the problem solving of any issues that may happen during the course of the study. Barring site staff, they ensure that the right protocols are being followed, and, in informing the sponsors and regulatory bodies, they ensure the continuity of the trial. In the case of CRAs, they ensure that there is an open and professional communication in order to develop a properly coordinated research process that will lead to the right data collection and patient safety.
Documentation
It is very important to maintain detailed and accurate records in a clinical research. CRAs document the entire trial, from the site visit reports to the protocol deviations, and the communication with the investigative sites. Documentation is important to ensure that the study is transparent, traceable and compliant with the regulatory standards. These records serve to prove that the trial did, in fact, adhere to all of the guidelines and can help to resolve any issues that may occur. In this way, CRAs support the integrity of the research, for the trial data to be reliable and meet the necessary requirements for approval, by maintaining well organized documentation.
These responsibilities demonstrate the vital role of Clinical Research Associates (CRAs) in ensuring the success of clinical trials. They are involved in each task thus helping in ensuring that accurate and reliable research data is obtained. They play a key role in supporting regulatory compliance, but they also play a key role in protecting patient safety. Clinical trials can only be reliable in producing results that can lead to new treatments and medical advancements, if such efforts are well done. The role of the CRAs is therefore very important in making sure that new therapeutic opportunities are available to patients, enhance healthcare and define the future of medicine.
Why Should You Consider A Career As A Clinical Research Associate?
Pursuing a career as a Clinical Research Associate (CRA) can be a rewarding choice for those interested in healthcare and medical advancements. CRAs play a vital role in ensuring clinical trials are conducted ethically and efficiently. With high demand, competitive salaries, and opportunities for career growth, this profession offers a stable and fulfilling path in the pharmaceutical and research industries.
High Demand For CRAs
The growing pharmaceutical industry has led to an increasing number of clinical research studies, creating a strong demand for Clinical Research Associates (CRAs). These professionals are essential for managing trials, ensuring that studies follow proper guidelines, and maintaining the accuracy of collected data. With clinical research expanding worldwide, CRAs have excellent job opportunities, competitive salaries, and a chance to contribute to the development of new medical treatments. Their role is important in making sure trials run smoothly while meeting safety and regulatory requirements.
Opportunities For Career Advancement
A career as a Clinical Research Associate (CRA) opens the door to many opportunities for growth and specialization. With experience, CRAs can move into leadership roles or focus on specific areas of clinical research. Some may become Clinical Trial Managers, supervising multiple studies and guiding research teams, while others may choose a path in Regulatory Affairs, ensuring that trials follow all necessary rules. Project Management is another option, where professionals oversee trials from beginning to end. These career paths not only provide professional growth but also allow CRAs to contribute to advancements in medical science and patient care.
Attractive Salary Packages And Benefits
A career as a Clinical Research Associate (CRA) offers a competitive salary along with valuable benefits. Entry-level CRAs start with good pay, and their earnings grow as they gain experience and certifications. In addition to salary, many CRAs receive health insurance, retirement savings plans, and paid time off, allowing them to maintain a balanced work-life routine. These financial and personal benefits make the role attractive to those looking for a stable and rewarding career. With continuous growth in the clinical research field, CRAs can expect long-term job security and opportunities for higher earnings.
The Path To Becoming A Successful Clinical Research Associate
Before stepping into a career as a Clinical Research Associate (CRA), it is important to evaluate if this profession matches your skills, interests, and long-term goals. Understanding the key aspects of the role will help you decide whether it is the right career path for you.
1. Assessing Your Suitability for the Role
Before starting a career as a Clinical Research Associate (CRA), it is essential to consider whether this profession suits you. Take a moment to reflect on your interests, skills, and qualifications to see if they align with the responsibilities of a CRA. Understanding your strengths and career goals will help you decide if this is the right path for you.
Exploring Your Interests
Interest In Medical Research: CRAs play a key role in clinical trials that contribute to medical advancements. If you are passionate about improving healthcare and working with scientific data, this role may be a good fit.
Attention To Detail: The job requires careful monitoring of trial procedures, data accuracy, and compliance with regulations. If you enjoy being thorough and precise, this role can be rewarding.
Adaptability And Problem-Solving: Clinical trials often present challenges such as protocol deviations or unexpected issues. Being adaptable and having strong problem-solving skills will help you succeed.
Evaluating Your Skills
Strong Communication: CRAs collaborate with site staff, sponsors, and regulatory teams. Being able to explain complex information clearly is essential.
Time Management And Organization: Handling multiple studies, documents, and deadlines requires strong organizational skills and the ability to manage priorities effectively.
Technical Knowledge: Familiarity with clinical trial software, regulatory guidelines, and data management systems is beneficial for tracking and verifying trial data.
Reviewing Your Qualifications
Educational Background: Most CRAs hold degrees in life sciences, pharmacy, nursing, or related fields. Ensuring your academic background aligns with industry expectations is important.
Clinical Research Experience: Previous experience, such as internships, volunteer work, or working in clinical trial settings, can provide a strong foundation for entering the CRA profession.
If you are looking to gain relevant experience in clinical research, exploring resources on clinical trial experience can be helpful. Additionally, those interested in expanding their knowledge in specialized areas, such as pharmacovigilance, can benefit from courses offered by industry forums. Conducting a thorough self-assessment will give you a clearer understanding of whether a career as a Clinical Research Associate (CRA) aligns with your strengths and goals. This step also helps in identifying the education and skills needed to succeed in the field.
2. Acquiring The Necessary Education And Skills
Becoming a Clinical Research Associate (CRA) requires a combination of education and practical skills. Since CRAs work in a highly regulated and detail-oriented field, they need to have a strong foundation in medical knowledge, research ethics, and regulatory requirements. Gaining the right qualifications and training will not only improve job prospects but also ensure that aspiring CRAs are well-prepared for the responsibilities of the role.
Importance Of An Academic Degree
A degree in Life Sciences, Biology, Pharmacy, Nursing, or Health Sciences is often a basic requirement for becoming a CRA. This educational background helps in understanding clinical trials, medical procedures, and drug development. Employers prefer candidates with formal education as it reflects their commitment and readiness for the field. Apart from a degree, some professionals choose to pursue specialized certifications in clinical research to strengthen their knowledge and improve career opportunities. Having the right academic foundation makes it easier to grasp complex medical concepts and regulatory guidelines, which are essential for success in this role.
Medical Terminology Knowledge
CRAs frequently deal with medical reports, trial data, and regulatory documents that contain technical terminology. A strong understanding of medical terminology is necessary to interpret and communicate research findings accurately. Misinterpretation of terms can lead to errors in data collection and reporting, which can affect clinical trial outcomes. To build this skill, aspiring CRAs can take specialized courses focused on medical terminology used in clinical research. Programs like those offered by CCRPS provide structured learning that enhances proficiency in medical language, ensuring better collaboration with healthcare professionals and regulatory bodies.
Understanding Regulations
Since clinical trials are conducted under strict local and international laws, CRAs must have a clear understanding of the guidelines that govern research practices. Regulations such as ICH GCP (International Council for Harmonisation Good Clinical Practice) ensure that trials are conducted ethically and that patient safety is maintained. Knowledge of these regulations helps CRAs navigate compliance requirements and avoid legal or ethical violations. Many training programs offer courses on regulatory affairs, providing CRAs with the necessary insights to ensure that clinical trials adhere to industry standards and best practices.
Gaining the right education and skills is essential for a successful career as a Clinical Research Associate. A relevant degree, medical terminology proficiency, and knowledge of research regulations form the core foundation for excelling in this field. Investing in education and specialized training will not only boost career opportunities but also help in handling the challenges of clinical research with confidence and efficiency.
3. Gaining Experience In The Field Of Clinical Research
Getting hands-on experience is essential for anyone pursuing a career as a Clinical Research Associate (CRA). Practical exposure not only strengthens your resume but also helps you understand the real-world responsibilities of the role, preparing you for future challenges.
Internships, Volunteer Work, And Certificate Courses
Internships and volunteer work at research organizations, hospitals, or pharmaceutical companies provide a great way to gain industry experience. These opportunities allow you to observe clinical trials, interact with professionals, and understand regulatory requirements. Enrolling in certificate courses focused on areas like pharmacovigilance and pharmacoepidemiology can further boost your knowledge. Programs from institutions such as CCRP offer structured training that makes you more competitive in the job market. Volunteering also demonstrates your commitment to clinical research, making you a stronger candidate for future roles.
Entry-Level Positions
Starting with entry-level roles like Clinical Trial Assistant or Data Coordinator can help build the experience needed to become a CRA. These positions involve tasks such as monitoring data quality, assisting in patient recruitment, and supporting senior researchers. Working in these roles allows you to develop essential skills while gaining an in-depth understanding of clinical trial processes. Employers value candidates with hands-on experience, and these jobs provide the foundation needed to transition into more advanced research roles.
Personal Assessment
Before stepping into clinical research, assessing your skills and interests can be beneficial. Consider whether you have strong organizational skills, attention to detail, and the ability to manage multiple tasks effectively. Since clinical trials require precision and compliance with strict regulations, these qualities play a key role in a CRA’s success. By understanding your strengths, you can determine if this career aligns with your professional goals. Gaining practical experience through internships, volunteer work, and entry-level jobs will help refine your skills and prepare you for a rewarding future in clinical research.
Benefits Of Becoming Certified
Better Job Opportunities: Many employers prefer certified CRAs because it proves their expertise and commitment to the field. Certification can make your job applications stronger.
Increased Professional Credibility: Having certification shows that you have the required skills and knowledge, making you more respected among employers and colleagues.
Career Growth: With certification, you can qualify for higher-level positions, including management roles or specialized areas in clinical research.
Investing in certification is a smart choice for those who want to build a strong career as a CRA. It not only improves your qualifications but also assures employers that you can handle complex clinical trials.
For those looking to expand their knowledge, CCRPS provides valuable resources. They offer insights into medical efficacy definitions and guidance on how to find clinical trials for cancer. These resources can help professionals stay informed and advance in the field.
Certifications do more than just enhance your resume—they open doors to new opportunities and professional development in the ever-evolving world of clinical research.
4. Nailing The CRA Job Application Process
Applying for a Clinical Research Associate (CRA) position requires careful preparation and a smart approach. Understanding the right strategies can help you stand out and increase your chances of securing the job.
Strategies For Finding And Applying For CRA Positions
1. Use Online Job Portals:
Search for CRA roles on websites like Indeed, Glassdoor, and LinkedIn.
Set up job alerts to get updates on new opportunities.
2. Expand Your Professional Network:
Join LinkedIn groups related to clinical research.
Attend industry conferences and networking events.
Connect with professionals through CCRPS’s alumni network for job leads and advice.
3. Create A Strong Resume And Cover Letter:
Resume Tips:
List your education, certifications, and work experience clearly.
Use relevant keywords from the job posting to pass Applicant Tracking Systems (ATS).
Add numbers to highlight achievements (e.g., "Assisted in 50+ clinical trials with a 98% compliance rate").
Cover Letter Tips:
Customize each cover letter to match the job role and company.
Show your understanding of clinical research and how your skills fit the position.
Mention any certifications or training from organizations like CCRPS.
Preparing For Interviews With Potential Employers
1. Research The Company:
Learn about their mission, values, and ongoing clinical trials.
Understand their focus areas, such as cancer drug research.
2. Demonstrate Your Knowledge:
Be ready to discuss clinical trial phases, Good Clinical Practice (GCP), and regulatory guidelines.
Provide examples of how you have applied your skills in real-world settings.
3. Prepare For Behavioral Questions:
Expect questions about teamwork, problem-solving, and handling challenges.
Use the STAR method (Situation, Task, Action, Result) to give structured answers.
4. Showcase Technical Skills:
Be familiar with industry software like Electronic Data Capture (EDC) systems.
Highlight any experience with tools used in clinical trial management.
By following these steps, you can present yourself as a well-prepared and competitive candidate in the clinical research field.
Participation In Workshops And Conferences
Workshops: Attending workshops helps CRAs gain practical experience and improve essential skills needed for clinical research. These sessions focus on important areas such as updated research methods, changes in regulations, and advancements in trial technologies. Hands-on activities allow participants to apply their knowledge in real-world scenarios, making them better prepared for industry challenges.
Conferences: Industry conferences offer valuable networking opportunities, bringing together professionals from different areas of clinical research. These events provide insights into the latest trends, including advancements in drug development, patient care, and compliance standards. Engaging with experts and peers helps CRAs expand their knowledge and stay updated on industry developments.
Ongoing Training Opportunities
Online Courses: Taking online courses is a convenient way for CRAs to keep up with industry standards and expand their knowledge. Platforms like CCRPS provide specialized certification programs, including CRA, CRC, and ICH GCP. These courses help professionals develop essential skills and stay updated on regulatory requirements, making them more competitive in the field.
In-House Training Programs: Many organizations offer internal training programs to ensure their staff is well-equipped with the latest industry practices. These programs help CRAs understand company-specific protocols, improve efficiency, and meet employer expectations, ultimately enhancing their performance in clinical research.
Self-Assessment And Personal Growth
Regular self-assessment is an important step for CRAs to recognize their strengths and identify areas where they can improve. By evaluating skills and setting career goals, professionals can find opportunities to specialize in fields like data management, regulatory affairs, or clinical trial monitoring. Staying informed about industry trends is equally important, and resources such as the clinical trial assistant salary guide can help professionals understand salary expectations and career growth opportunities. Additionally, reviewing clinical trial monitoring plan SOPs can provide valuable insights into best practices. Continuous learning and self-improvement not only increase competency but also open doors to better career prospects in the ever-evolving field of clinical research.
How CCRPS Can Help You Become A Clinical Research Associate Faster?
Comprehensive Online Certification Courses
CCRPS offers comprehensive online certification courses tailored to help aspiring Clinical Research Associates (CRAs) develop essential skills. These programs cover key industry topics, ensuring a strong foundation for a successful career in clinical research.
Benefits Of Choosing Online Learning
Enrolling in an online certification program through CCRPS offers several key benefits.
Flexibility: Online courses allow you to study at your own pace, making it easier to balance learning with work or other responsibilities. You can set your own schedule and progress in a way that suits you best.
Convenience: With online access, you can learn from anywhere without the need for travel or relocation. This makes it easier to complete your certification while managing your daily commitments.
Another major advantage is the ability to revisit lessons whenever needed. This ensures you fully understand each topic before moving forward, helping you build a solid foundation in clinical research.
Ensuring Industry Relevance Through Updated Curriculum
CCRPS continuously updates its courses to align with the latest industry standards and regulatory requirements. By integrating new clinical research techniques and evolving guidelines, the curriculum ensures students gain relevant and up-to-date knowledge. This approach helps graduates stay competitive in the job market and confidently handle the challenges of clinical research. Many graduates have successfully transitioned into CRA roles, crediting CCRPS training to give them a strong foundation. The comprehensive coursework not only enhances their expertise but also improves job prospects by preparing them for interviews and real-world clinical trials, making them valuable assets in the field.
Recognition And Trust In The Field
CCRPS is a well-respected training provider in the clinical research industry, known for its high-quality certification programs. Many top research organizations value candidates with CCRPS certification, recognizing their strong knowledge and skills. This industry trust not only enhances your resume but also strengthens your credibility as a CRA. Choosing CCRPS means joining a reputable institution that consistently produces well-trained professionals. The comprehensive training ensures you are well-prepared for career opportunities, giving you a competitive edge in the field. Earning a certification from CCRPS is a smart step toward a successful future in clinical research.
Conclusion
Pursuing a career as a Clinical Research Associate (CRA) opens doors to professional growth, competitive salaries, and the opportunity to contribute to medical advancements. While the journey may seem challenging, the right training and dedication can help you succeed. CCRPS provides comprehensive online certification programs designed to equip aspiring CRAs with essential knowledge and skills. These courses offer flexibility, allowing you to learn at your own pace while ensuring you stay updated with the latest industry standards. Earning a CCRPS certification enhances your professional credibility, giving you a competitive edge in the field and helping you achieve long-term career success.
With CCRPS resources, you can transform your career aspirations into reality. Join thousands of successful graduates who have gained the skills and knowledge needed to excel in clinical research. Contact us today and take the next step toward becoming a Clinical Research Associate.
FAQs (Frequently Asked Questions)
-
A Clinical Research Associate (CRA) monitors and oversees clinical trials for pharmaceutical companies, contract research organizations (CROs), or medical institutions. Their job is to ensure that trials follow all regulatory guidelines, study protocols, and Good Clinical Practice (GCP) standards. They also check the accuracy of trial data and collaborate with site staff to resolve any issues that arise during the study.
-
A career as a Clinical Research Associate offers strong job opportunities, career growth, and competitive salaries. As the demand for clinical research continues to grow, CRAs are in high demand. This role provides not only financial benefits but also opportunities to advance into management positions or specialize in different research areas.
-
To become a successful CRA, you need to take a few key steps:
Evaluate your skills and interests to see if the role suits you.
Get the right education in Life Sciences or a related field and learn medical terminology.
Gain hands-on experience through internships or entry-level roles.
Consider certification from reputable organizations like CCRPS to strengthen your qualifications.
Prepare for job applications by crafting a strong resume and practicing for interviews.
Continue learning through training programs and industry updates to stay ahead in the field.
-
CCRPS offers online certification courses designed for aspiring CRAs. These courses cover essential topics such as CRA responsibilities, CRC training, and ICH GCP guidelines. With CCRPS, you can learn at your own pace while gaining the knowledge and skills needed to enter the field. Their courses stay up to date with industry trends, making it easier for you to get hired and succeed in your career.