The Ultimate Guide to Getting Your Good Clinical Practice (ICH-GCP) Certification in UK: Everything You Need to Know in 2025
In the UK, Good Clinical Practice (GCP) certification is a mandatory regulatory requirement for anyone involved in clinical trials. From Clinical Research Nurses and CRCs at NHS Trusts to CRAs, CTAs, and Principal Investigators working on NIHR or MHRA-regulated studies—ICH-GCP training is a non-negotiable prerequisite. Without it, your CV won't pass through NHS R&D governance, IRAS submissions will stall, and trial sponsors will not delegate responsibilities to you. Yet many professionals still rely on outdated GCP slide decks that don’t cover current ICH E6(R3) changes, risk-based monitoring, or AE classification.
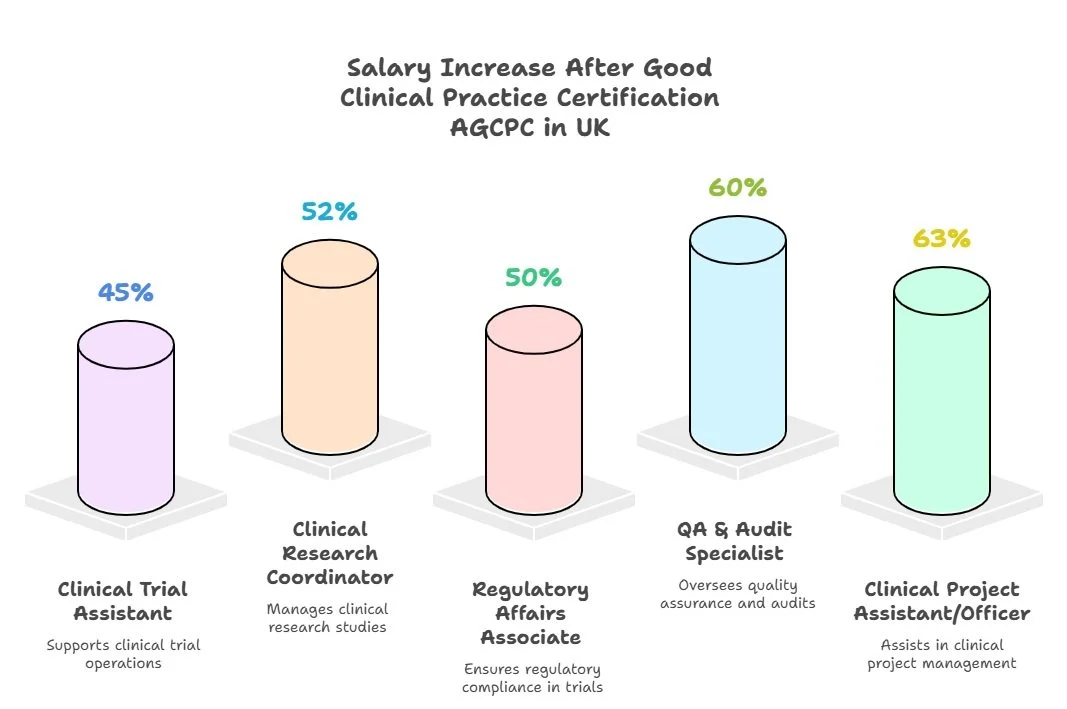
A proper GCP certification doesn’t just ensure compliance—it directly increases your earning potential by 30%–55%, especially in sponsor-facing roles. Whether you're aiming for positions at IQVIA UK, NHS Research Delivery Networks, AstraZeneca, or academic trials at UCL, advanced ICH-GCP certification proves you're audit-ready and protocol-competent. With MHRA inspections tightening in 2025, clinical professionals must now show proficiency in CAPA planning, remote trial protocols, and patient safety documentation. Without it, you're not just underqualified—you’re unqualified to lead or even support modern UK-based clinical research.
What Is Good Clinical Practice (ICH-GCP) Certification Online in the UK Exactly? Skills Required and Jobs Explained
In the UK, Good Clinical Practice (GCP) certification is a legal and ethical prerequisite for anyone involved in human clinical trials. Whether you're based at an NHS Trust site, working for a commercial CRO, or preparing MHRA submissions, ICH-GCP training aligned with E6(R3) and E8(R1) is required. But not all certifications prepare you for real inspection-readiness. A modern UK-aligned GCP course must train you on HRA/IRAS workflows, AE/SAE documentation, eConsent protocols, and decentralised trial oversight—not just ethics principles.
A proper ICH-GCP certification online helps professionals secure compliance in MHRA-regulated trials, NIHR multicentre studies, and NHS R&D audits. It equips you to lead CAPA development, respond to MHRA inspections, align with 21 CFR Part 11 for eSystems, and manage trial conduct across hybrid and remote trial models. Below is a detailed table of UK clinical roles and the practical skills GCP certification delivers.
What Skills Does a Certified Good Clinical Practitioner in UK Actually Gain?
GCP Compliance Mastery – Understand and apply ICH E6(R3), E8(R1), and local regulations
Informed Consent Execution – Document, deliver, and verify consent processes
AE/SAE Reporting – Classify, document, and escalate adverse events accurately
21 CFR Part 11 Adherence – Ensure eSignatures, audit trails, and data integrity standards
Protocol Deviation Management – Identify, log, and report deviations with impact analysis
CAPA Planning & QA Readiness – Build inspection-ready CAPAs, prepare for FDA/EMA/MHRA audits
Decentralized Trial Oversight – Run eConsent, telehealth visits, and remote monitoring protocols
Regulatory Filing Prep – Package IRB/EC submissions, manage SOP-aligned documents
Mock Audit & Inspection Tools – Navigate GCP inspection scenarios using real deliverables
Digital Trial Compliance – Monitor compliance in EDC, eTMF, and RBM-enabled trials
What Jobs Can You Apply for With ICH-GCP Certification in UK?
Clinical Research Associate (CRA) (IQVIA UK, Syneos Health UK, PRA Health Sciences, Parexel London)
Clinical Trial Assistant (CTA) (ICON Plc UK, Clario, Veristat, Richmond Pharmacology)
Clinical Research Coordinator (CRC) (NHS Trusts, CRN North Thames, UCLH Biomedical Research Centre)
Principal Investigator (PI) (Required across: Oxford University Hospitals, NHS Digital Trials Units, King's College London)
Regulatory Affairs Associate (AstraZeneca UK, GSK, NIHR Regulatory Support Centres)
QA & Compliance Lead (NHS R&D Units, AstraZeneca Macclesfield, NHS Lothian Clinical Trials Unit)
Decentralized Trial Specialist (Medable UK, Science 37, NIHR Innovation Observatory, ICON Digital Trials)
Safety Monitor / Data Manager (Clario UK, NHS Pharmacovigilance Teams, Cambridge Clinical Trials Unit)
Why Should You Get GCP Certification to Work in the UK?
In the UK, ICH-GCP certification is not optional—it’s enforced across MHRA-regulated studies, NIHR-supported trials, and NHS R&D departments. Without it, professionals are excluded from delegated trial responsibilities such as informed consent, AE documentation, or IRAS submissions. These restrictions limit salaries to £24K–£30K and cap progression at entry-level assistant roles. With a proper GCP credential, however, professionals can access roles across clinical QA, ethics coordination, site leadership, and regulatory liaison, with average compensation rising to £35K–£55K+. Organisations like AstraZeneca UK, IQVIA, and NHS Research Delivery Network require this certification for onboarding, audit participation, and compliance clearance.
| Aspect | Without GCP Certification | With GCP Certification |
|---|---|---|
| Typical Salary Range | £24K–£30K | £35K–£55K+ |
| Allowed Responsibilities | Basic admin support, no AE/consent handling | Consent, AE logs, HRA/IRAS documentation |
| Audit Participation | Restricted or non-participatory | Lead CAPA, SOP review, inspection readiness |
| Shortlisting for Roles | Filtered out by NHS R&D and CROs | Required for PI delegation and CRO contracts |
| Career Path Mobility | Stuck in assistant/support tiers | Open to CRA, QA, regulatory, and DCT leadership roles |
Which Certification Should You Choose to Get GCP Certified in the UK?
In the UK, professionals often choose outdated NHS online modules or brief sponsor-led GCP refreshers that meet the “tick box” requirement—but lack real-world application. These options rarely cover MHRA audit scenarios, CFR Part 11, or decentralized trial processes, and almost none include tools like AE classification templates or CAPA frameworks. Without these, professionals may be certified—but not ready. NHS trusts and CROs increasingly seek GCP-trained staff who can operate autonomously in HRA, IRAS, and ICH E6(R3) environments.
By contrast, the Advanced Good Clinical Practice Certification (AGCPC) by CCRPS is built for frontline UK professionals. With 70+ modules, 70 CPD/CME credits, TransCelerate recognition, and built-in 2-year refreshers, it doesn’t just certify—it prepares. Whether you’re applying at NHS R&D, AstraZeneca UK, or Clario, AGCPC proves you’re ready for trial delivery, not just ethics theory. It’s audit-tested, inspection-aligned, and trusted by alumni placed at NIHR, J&J, NHS Trusts, and CROs.
| Comparison Point | Other GCP Courses (UK) | CCRPS AGCPC Certification |
|---|---|---|
| Accreditation | Basic sponsor/NHS acknowledgment | CPD, CME (70 hrs), TransCelerate-recognized |
| Curriculum Depth | 2–4 hour ethics overviews | 70+ modules, real AE/SAE casework, RBM, DCT, CAPA |
| Format & Flexibility | Static PDFs or slide-based LMS | 100% online, lifetime access, 2-year refresher built-in |
| Tools & Templates | Minimal—no downloadable tools | AE tables, CAPA forms, eConsent kits, mock audit tools |
| Instructor Access | None or generic support inbox | Real-world mentors, course creators available |
| Certificate Format | PDF download only | High-res PDF, LinkedIn badge, blockchain-verifiable URL |
Why CCRPS’s AGCPC Will Be a Game Changer for Your Career in the UK
UK-based clinical professionals who complete AGCPC are transitioning from entry-level support roles to sponsor-facing positions in trial delivery, QA, ethics governance, and decentralized trial operations. Before certification, roles like CRC or CTC are capped at £26K–£32K, with limited autonomy. But with AGCPC, professionals enter positions commanding £38K–£60K+, often within 6–12 months. Employers like NHS R&D units, AstraZeneca UK, Syneos Health, and UCL CTUs seek GCP-certified staff to lead IRAS/HRA prep, audit response, AE oversight, and hybrid protocol rollout. AGCPC isn’t just proof of training—it’s evidence of inspection readiness.
Section 6 – Summarizing All You Need to Know About Getting Your AGCPC in the UK
If you're planning to work on MHRA-regulated, HRA-approved, or NIHR-funded trials, AGCPC is not just a certificate—it’s your license to operate. From CRA and CRC roles to regulatory, QA, or decentralized trial leadership, CCRPS’s AGCPC provides tools, credentials, and inspection readiness aligned with UK clinical systems. It gives NHS, CRO, and sponsor trial staff the ability to manage informed consent, AE/SAE documentation, and MHRA-facing deliverables—supported by a lifelong credential and real-world templates.
| Factor | Details |
|---|---|
| Certification Name | Advanced Good Clinical Practice Certification (AGCPC) |
| Accreditations | CPD, CME (70 hours), TransCelerate-recognized |
| Format | 100% online, self-paced, lifetime access + 2-year refreshers |
| Built For | CRCs, CTAs, CRAs, QA Officers, NHS Trial Staff |
| Tools Included | AE tables, eConsent kits, audit templates, CAPA forms |
| Exam | Proctored final exam, 2 attempts, 70% pass required |
| Credential Issued | PDF + LinkedIn badge + blockchain-verifiable certificate |
| Recognized By | AstraZeneca UK, NHS R&D, IQVIA, UCL CTUs |
Frequently Asked Questions
-
Yes. AGCPC is recognized by clinical research employers across the UK, especially for MHRA-regulated trials and NIHR-funded studies. NHS R&D departments, CROs like IQVIA UK and Syneos, and academic sponsors at UCL or Oxford now require formal GCP documentation for protocol-facing staff. AGCPC’s CPD and CME accreditations, 70+ modules, and TransCelerate recognition make it more than acceptable—it positions you for delegation, audit involvement, and trial oversight roles. NHS trusts may also ask for evidence of refresher training, which AGCPC includes every two years at no extra cost.
-
Absolutely. Most UK-based CRAs and QA officers were previously CRCs or CTAs. AGCPC fills the exact skills gap: AE logging, CAPA planning, IRAS documentation, and inspection readiness. After certification, many alumni report transitioning into CRA, QA coordinator, or trial manager roles with salary boosts of £8K–£15K annually. The course also includes templates (CAPAs, audit kits) that you can use directly in your role. Certification alone won’t guarantee promotion—but AGCPC gives you the operational skills needed to qualify and stand out in interviews.
-
You can complete the course in as little as 3–4 weeks if studying full-time, or over 6–8 weeks part-time alongside NHS or CRO work. All 70+ modules are fully online and self-paced, with no expiration date. You’ll have lifetime access, along with free refresher updates every two years as ICH-GCP guidelines evolve. There’s also a final proctored exam (with two attempts included) that validates learning and generates your certificate, LinkedIn badge, and blockchain URL.
-
Yes. The certificate is recognized by MHRA auditors, HRA governance units, and commercial sponsors. It’s issued as a high-resolution PDF, a blockchain-verifiable credential URL, and a shareable LinkedIn badge. Many NHS and CRO hiring managers now ask for verifiable credentials beyond basic PDFs—AGCPC meets that standard. It also meets TransCelerate standards for GCP training in sponsor documentation, which increases credibility when working on global protocols or EU/US multicentre studies.
-
You’ll get full access to real-world instructors, not just static slides. There are 1-on-1 mentorship options, live support channels, and downloadable walkthroughs for tough topics like CAPA, AE classification, and Part 11 compliance. Many learners also return to reuse the AE templates, protocol deviation logs, and SOP tools included. If you ever face an MHRA audit, you’ll already be equipped with inspection-aligned content and response formats used in NHS and CRO settings.
Sources: Whitehall Training Blog, STG Talent