Top 20 Terms Every Clinical Research Coordinator CRC Must Understand Clearly
Mastering clinical research terminology is a fundamental requirement for Clinical Research Coordinators (CRCs). The role of a CRC is not merely administrative but pivotal in ensuring regulatory compliance, data integrity, and patient safety. Precision in understanding terms—from source document verification to adverse event reporting—directly impacts trial success. Each term signifies a protocol, regulation, or standard that must be followed to the letter. CRCs are tasked with bridging communication gaps between sponsors, investigators, and regulatory bodies, making it essential to decode and apply clinical research terminology without ambiguity.
Failure to grasp even a single term can cause protocol deviations, delay approvals, and compromise study data. In an environment driven by global regulatory authorities like the FDA, EMA, and MHRA, CRCs cannot afford to rely on guesswork. This article presents a detailed, table-based list of 20 critical terms, each with its precise definition and real-world relevance. Every term will be explained not just for its meaning but for its practical application in the daily responsibilities of a Clinical Research Coordinator. Additionally, this guide will offer actionable strategies to ensure CRCs are equipped to navigate complex protocols confidently, minimizing errors and enhancing trial outcomes.
Essential Terminology for Clinical Research Coordinators
Clinical trials operate within a rigorously structured environment where clarity and precision in language are essential. As a Clinical Research Coordinator (CRC), mastering core terminology is more than academic—it’s a strategic imperative. Each term encapsulates a regulation, a process, or a risk mitigation strategy that can determine trial outcomes. CRCs must interpret, apply, and communicate these terms with precision to ensure smooth operations and compliance.
This section introduces a table of the 20 most critical terms every CRC must understand and apply. These terms are not random—they represent the core pillars of clinical research practice. For each, we provide a crisp definition and its direct relevance to a CRC’s role. Mastery of these terms will enhance data quality, reduce compliance risks, and sharpen a CRC’s competitive edge.
| Term | Definition | Practical Application/Importance for CRCs |
|---|---|---|
| Source Document Verification (SDV) | The process of comparing data recorded in case report forms (CRFs) with original source documents to ensure accuracy and completeness. | Ensures data integrity, identifies discrepancies, and minimizes regulatory audit risks. |
| Informed Consent Form (ICF) | A document detailing study procedures, risks, and rights, signed by participants. | Secures legal and ethical compliance, ensures participant autonomy, and supports audit readiness. |
| Case Report Form (CRF) | A standardized form for capturing clinical trial data. | Facilitates accurate data collection, simplifies data management, and enables regulatory reporting. |
| Serious Adverse Event (SAE) | Any untoward medical occurrence resulting in significant harm or requiring intervention. | Triggers immediate reporting, ensuring regulatory compliance and participant safety. |
| Clinical Trial Protocol | A document outlining study objectives, design, methodology, and analysis plan. | Guides CRCs’ daily operations, defines inclusion/exclusion criteria, and ensures consistency across sites. |
| Good Clinical Practice (GCP) | An international ethical and scientific quality standard for designing, conducting, and reporting clinical trials. | Establishes operational benchmarks, safeguards participant rights, and ensures data credibility. |
| Regulatory Binder | A collection of essential documents demonstrating study compliance. | Organizes trial documentation, supports regulatory audits, and enhances transparency. |
| Monitoring Plan | A document detailing the strategy for trial monitoring. | Defines oversight procedures, clarifies monitoring frequency, and mitigates risks. |
| Data Clarification Form (DCF) | A form used to query discrepancies in data collected during a trial. | Streamlines data query resolution, ensures data accuracy, and reduces delays. |
| Protocol Deviation | Any instance where the approved protocol is not followed. | Identifies and reports non-compliance, maintains study validity, and minimizes audit findings. |
| Subject Screening Log | A record of potential participants evaluated for eligibility. | Tracks recruitment efforts, documents inclusion/exclusion decisions, and supports enrollment targets. |
| Audit Trail | A chronological record of changes made to electronic data. | Ensures data traceability, supports regulatory audits, and confirms data integrity. |
| Randomization | The process of assigning study participants to treatment groups by chance. | Reduces selection bias, enhances study validity, and supports regulatory approval. |
| Electronic Data Capture (EDC) | A system for electronic collection of clinical trial data. | Improves data accuracy, reduces transcription errors, and accelerates data availability. |
| Investigator Brochure (IB) | A compilation of clinical and non-clinical data on the investigational product. | Informs study teams, supports regulatory submissions, and guides trial conduct. |
| Confidentiality Agreement | A legal document protecting sensitive trial information. | Secures proprietary data, prevents unauthorized disclosure, and upholds compliance. |
| Site Initiation Visit (SIV) | A pre-study visit to prepare the site for the trial. | Ensures site readiness, clarifies roles and responsibilities, and mitigates startup delays. |
| Screen Failure | A participant who fails to meet eligibility criteria. | Prevents protocol violations, documents screening efforts, and supports enrollment reporting. |
| Interim Analysis | A review of trial data before study completion. | Identifies early trends, supports decision-making, and manages risk. |
| Final Study Report | A comprehensive report summarizing trial results. | Documents study outcomes, supports regulatory submissions, and enhances transparency. |
Regulatory Context of CRC Terminology
Precise terminology usage is not optional for Clinical Research Coordinators (CRCs)—it’s a regulatory mandate. Regulatory frameworks, spearheaded by global authorities, demand unambiguous communication to ensure trial data integrity, participant safety, and legal compliance. Failure to apply these terms accurately can trigger regulatory actions, jeopardizing not just the study but also an organization’s reputation and operational license.
Good Clinical Practice (GCP) and Terminology Mastery
The cornerstone of CRC terminology compliance is Good Clinical Practice (GCP)—a universally recognized standard that governs clinical trial design, conduct, and reporting. GCP establishes a clear framework for trial processes, where terminology reflects specific obligations and risk management protocols. Every critical term, from source document verification to SAE reporting, is codified within GCP to ensure data credibility, patient protection, and regulatory approval readiness. CRCs who master GCP-defined terminology can preempt common compliance issues, streamline documentation, and significantly reduce site audit findings. This mastery translates directly to operational excellence, accelerating trial timelines and minimizing costly delays.
Common Regulatory Bodies and Standards
Understanding the global regulatory landscape is vital for a CRC. Authorities such as the FDA, EMA, and MHRA enforce distinct standards that shape terminology requirements across jurisdictions. The FDA mandates detailed documentation and reporting protocols, while the EMA focuses on harmonization through ICH guidelines. The MHRA, aligned with UK regulatory frameworks, imposes rigorous compliance checks, particularly in the post-Brexit era. CRCs must adapt terminology comprehension to meet these evolving standards, ensuring seamless operations regardless of geographic scope. Mastery of terminology aligned with these regulators is a competitive advantage, positioning CRCs to support global trials effectively.
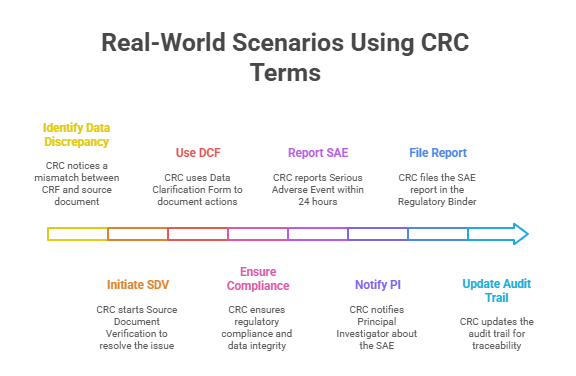
Real-World Scenarios Using CRC Terms
Case Study 1: Handling Source Data Discrepancies
A routine monitoring visit reveals a mismatch between the data recorded in the Case Report Form (CRF) and the original source document—a common scenario in clinical trials. The CRC immediately initiates Source Document Verification (SDV) to confirm the correct data. Upon investigation, the discrepancy arises from a transcription error during data entry. The CRC documents the discrepancy using a Data Clarification Form (DCF), detailing the error, the corrective action, and the verification of accurate data entry. This immediate, precise response ensures regulatory compliance and maintains data integrity.
The CRC’s familiarity with SDV and DCF protocols streamlines resolution, avoiding potential protocol deviations. Additionally, a proactive approach during monitoring visits minimizes findings during regulatory audits and enhances sponsor confidence. Mastery of these terms not only facilitates swift issue resolution but also demonstrates a robust understanding of quality control principles in clinical research. This case underscores the critical need for CRCs to internalize core terminology and apply it seamlessly in real-world settings.
Case Study 2: SAE Reporting Protocol
A participant in a multi-center oncology trial experiences a Serious Adverse Event (SAE) requiring hospitalization. The CRC’s immediate priority is to notify the Principal Investigator (PI) and initiate the site’s SAE reporting protocol. Leveraging their understanding of GCP guidelines and site-specific Monitoring Plan requirements, the CRC prepares the SAE report, capturing event specifics, patient impact, and corrective actions. The report is submitted to the sponsor and regulatory authorities within the mandated 24-hour window.
To ensure audit-readiness, the CRC meticulously files the SAE report in the Regulatory Binder and updates the trial’s audit trail for traceability. This prompt, protocol-driven response not only ensures participant safety but also demonstrates rigorous adherence to compliance requirements. A solid grasp of SAE terminology empowers the CRC to act decisively, minimizing regulatory risk and reinforcing trust with sponsors and regulatory bodies. In high-stakes scenarios, terminology mastery directly translates to trial integrity and operational excellence.
Tools and Resources for Mastering CRC Vocabulary
Top CRC Glossaries and Handbooks
Clinical Research Coordinators aiming for terminology mastery must leverage the best reference materials. Essential resources include the ICH GCP Guidelines, which outline fundamental clinical research concepts and compliance standards. The FDA’s Glossary of Terms offers clear definitions directly linked to U.S. regulatory expectations, while the EMA’s EudraLex Volume 10 provides essential European regulatory context. The NIH’s Clinical Research Handbook also consolidates key terms into an accessible format, offering comprehensive explanations of critical concepts.
These glossaries and handbooks do more than define—they contextualize terminology for daily CRC application. They bridge the gap between theory and practice, enabling CRCs to align site-level documentation with global regulatory requirements. Utilizing these references consistently can reduce documentation errors, improve audit readiness, and strengthen professional credibility in competitive clinical environments.
Advanced E-Learning and On-the-Job Aids
Mastery of clinical research terminology extends beyond reference materials. CRCs should engage with interactive e-learning modules offered by recognized providers such as TransCelerate, ACRP, and CCRPS. These platforms offer scenario-based learning, quizzes, and practical assessments that reinforce terminology in real-world contexts. On-the-job aids like standard operating procedures (SOPs), digital glossary apps, and integrated EDC platform tooltips provide immediate access to definitions during live trials.
Regular engagement with these resources fosters continuous learning and error-free documentation. By integrating advanced learning tools with daily workflows, CRCs can stay current with evolving industry terms, mitigate compliance risks, and improve overall data quality. The combination of structured e-learning and real-time tools creates a comprehensive strategy for sustained terminology proficiency.
Career Impact of Strong CRC Terminology Knowledge
Enhanced Data Quality and Compliance
Mastering core CRC terminology elevates both individual and organizational performance. Precise understanding of terms like source document verification, informed consent, and protocol deviations reduces errors, streamlines data management, and fortifies compliance. In an industry where regulatory scrutiny is relentless, accurate terminology usage minimizes audit findings, prevents costly protocol amendments, and expedites study approvals.
CRCs with a solid terminology foundation actively contribute to seamless trial operations. Their ability to ensure regulatory compliance and maintain data integrity makes them indispensable to clinical sites and sponsors alike. This expertise directly impacts trial outcomes, fosters investigator trust, and drives operational excellence. Strong terminology skills enable CRCs to handle complex trials efficiently, maintaining high data quality and accelerating time-to-market for investigational products.
Competitive Career Advancement
CRCs with expert terminology proficiency distinguish themselves in a competitive job market. Sponsors, Contract Research Organizations (CROs), and clinical sites value professionals who can confidently navigate regulatory requirements and translate them into daily operations. Strong command of CRC-specific terms like SAE reporting, EDC systems, and regulatory binder management signals readiness for advanced roles, including lead CRC, clinical project manager, or regulatory specialist.
Additionally, terminology mastery opens doors to higher-paying roles and international opportunities. CRCs who demonstrate comprehensive knowledge in terminology can mentor junior staff, influence site policies, and contribute to process improvements, further elevating their professional standing. In an era of global trials and cross-jurisdictional complexity, this expertise is a strategic asset, propelling CRCs toward sustained career growth.
| Aspect | Description |
|---|---|
| Enhanced Data Quality and Compliance | Mastery of core CRC terminology—like source document verification, informed consent, and protocol deviations—reduces errors, streamlines data handling, and fortifies compliance. This proactive approach minimizes audit findings, expedites regulatory approvals, and drives operational excellence. |
| Regulatory Compliance and Data Integrity | Accurate terminology usage ensures seamless compliance with global standards, fostering sponsor and investigator trust. CRCs with a solid terminology foundation contribute directly to high-quality trial outcomes and enhanced data reliability. |
| Operational Efficiency | Strong terminology skills empower CRCs to manage complex trials confidently. Clear communication and accurate documentation reduce errors, shorten timelines, and improve overall trial performance, ensuring faster data readiness and submission. |
| Competitive Career Advancement | Proficiency in key terms like SAE reporting, EDC systems, and regulatory binder management positions CRCs for leadership roles—such as Lead CRC, Clinical Project Manager, or Regulatory Specialist—and increases marketability. |
| Professional Growth and Mentorship | Mastery of terminology enables CRCs to mentor junior staff, influence site policies, and implement process improvements, elevating their professional profile and driving long-term career success. |
| Global Career Opportunities | With terminology expertise, CRCs can confidently navigate complex, cross-jurisdictional trials, opening doors to high-value roles in global organizations and diverse regulatory environments. |
Why CCRPS-Certified CRCs Are Fluent in Clinical Research Terminology
The Clinical Research Coordinator Certification by CCRPS is meticulously designed to equip professionals with deep knowledge of essential clinical research terminology. This certification goes beyond basic definitions, delivering real-world, scenario-based mastery of critical terms like source document verification, SAE reporting, and GCP compliance. Through immersive modules, learners internalize the precise application of these terms in trial settings, ensuring immediate readiness for regulatory and sponsor expectations.
CCRPS’s curriculum integrates international regulatory frameworks—including FDA, EMA, and MHRA standards—ensuring learners can seamlessly adapt to global trials. The course features advanced EDC platform simulations, rigorous assessments, and interactive workshops, reinforcing terminology retention and practical skills. Graduates emerge as operationally proficient CRCs, capable of navigating complex trials with confidence and precision.
The certification’s value extends beyond technical knowledge. It elevates professional credibility, enhances employability, and positions CRCs as indispensable assets in competitive clinical environments. Employers recognize the CCRPS Clinical Research Coordinator Certification as a benchmark for excellence, opening doors to lead CRC positions, regulatory affairs, and clinical project management roles.
To take the next step in your career and master critical CRC terminology, explore the CCRPS Clinical Research Coordinator Certification course today. Equip yourself with the skills, knowledge, and confidence to excel in clinical research and set yourself apart as a leader in the field.
Frequently Asked Questions
-
Source Document Verification (SDV) stands as one of the most critical terms for CRCs aiming to ensure data integrity. SDV involves comparing data in the Case Report Form (CRF) to original source documents, confirming accuracy and completeness. Mastery of SDV not only safeguards against transcription errors and protocol deviations but also fortifies data credibility during regulatory audits. This process ensures that all recorded data reflect the real-world patient experience, a non-negotiable standard in clinical research. Proficiency in SDV allows CRCs to prevent discrepancies, support sponsor and regulatory confidence, and maintain the integrity of clinical trial data throughout the study lifecycle.
-
Good Clinical Practice (GCP) sets the benchmark for ethical, scientific, and operational standards in clinical trials. For CRCs, GCP compliance guides every aspect of trial conduct—from participant interactions to data management and reporting. Mastery of GCP terminology ensures CRCs maintain consistent documentation, informed consent processes, and accurate reporting of adverse events. Non-compliance with GCP can lead to protocol deviations, regulatory findings, or even trial suspension. By embedding GCP principles into daily workflows, CRCs uphold participant rights, ensure data validity, and align site practices with international standards, all while reducing operational risks.
-
Source Document Verification (SDV) is essential because it ensures the integrity of data collected during clinical trials. By meticulously comparing case report entries against original documents, CRCs identify errors, omissions, and discrepancies early. This proactive approach prevents inaccurate data from progressing into database lock and final analyses. Regulatory bodies such as the FDA and EMA require SDV to validate trial data and assess overall study credibility. Failure in SDV can delay regulatory approvals or trigger costly audit findings. For CRCs, strong SDV practices protect participant safety, enhance trial credibility, and support seamless submissions.
-
CRCs can utilize an array of tools to stay informed on evolving clinical research terminology. Key resources include the ICH GCP Guidelines, FDA Glossary, EMA’s EudraLex Volume 10, and the NIH Clinical Research Handbook, each providing structured terminology definitions and compliance expectations. Advanced e-learning platforms such as CCRPS, ACRP, and TransCelerate offer interactive modules with scenario-based learning and assessments. On-the-job tools like integrated Electronic Data Capture (EDC) platform glossaries, digital SOPs, and subscription-based regulatory updates ensure CRCs remain current. Regularly engaging with these resources enhances terminology mastery, strengthens compliance, and improves operational precision.
-
Terminology mastery aligns CRCs with the specific expectations of global regulatory authorities like the FDA and EMA. These bodies mandate precise documentation, including accurate descriptions of adverse events, protocol deviations, and participant consent. Misunderstood or misapplied terms can result in compliance failures, audit findings, or trial delays. CRCs proficient in core terminology are equipped to meet regulatory requirements seamlessly, reducing error rates and enhancing sponsor confidence. Mastery ensures that reporting, documentation, and trial conduct reflect the highest compliance standards, essential for gaining and maintaining regulatory approvals across jurisdictions.
-
Precise terminology is vital when managing Serious Adverse Events (SAEs). A CRC must recognize an SAE promptly, classify it accurately, and initiate the correct reporting process. Mastery of terms such as adverse event (AE), unexpected adverse drug reaction (UADR), and causality assessment ensures clear communication with investigators, sponsors, and regulatory bodies. This precision safeguards participant safety, supports compliance, and protects trial integrity. Failure to use standardized terminology can delay reporting, risk regulatory findings, and compromise patient well-being. Strong SAE terminology knowledge empowers CRCs to manage critical events efficiently and uphold high compliance standards.
-
The CCRPS Clinical Research Coordinator Certification offers comprehensive training focused on real-world terminology mastery. Its curriculum integrates interactive modules, practical assessments, and global regulatory frameworks including FDA, EMA, and MHRA standards. Participants gain hands-on experience with essential terms such as source document verification, GCP compliance, and SAE reporting through simulations and case studies. This immersive approach ensures not just academic understanding but also practical application in trial settings. Graduates are fully equipped to navigate complex protocols, reduce compliance risks, and enhance operational efficiency, positioning them as valuable assets in competitive clinical environments.
Final Thoughts
Mastering terminology is not optional for Clinical Research Coordinators—it’s the bedrock of trial success. Each term, from source document verification to GCP compliance, represents a critical standard that safeguards data integrity, participant safety, and regulatory alignment. CRCs who invest in terminology proficiency become invaluable to their sites and sponsors, driving compliance, accelerating approvals, and minimizing risk.
The Clinical Research Coordinator Certification by CCRPS is your direct path to mastering these essential terms. This certification not only teaches definitions but also embeds real-world application through immersive modules and advanced assessments. By completing this program, you’ll gain the skills, knowledge, and confidence to excel in clinical research and stand out in a competitive field.
Take the next step in your CRC journey—enroll in the CCRPS Clinical Research Coordinator Certification and transform your expertise into a career advantage today.