Understanding the Scope of Clinical Research and What it Entails
Clinical research can be defined as a type of research conducted on people to know about their ill and healthy condition. This is how we find out ways of preventing illnesses, making a diagnosis of diseases or even treating them. The aim is often to increase understanding and developing and validating new approaches to diagnosis and care to improve outcomes for patients.
In clinical research, a study protocol is honoured as being comprehensive and systematic. A clinical research can only be fully realised under certain conditions.
The research must:
Ensure that you gather the necessary consent from all the participants of the research.
Make sure that all the ethical steps are adhered to and all the approvals as required by the law are obtained.
Everyone who joins in the research, must have the necessary measures taken to protect them.
Be conducted by people who are competent.
Have the aim of growing medical knowledge.
Types of clinical research
There are two types of clinical research that can be conducted:
1. Observational studies (epidemiology, cohort study)
2. Clinical trials or Intervention studies
Observational studies
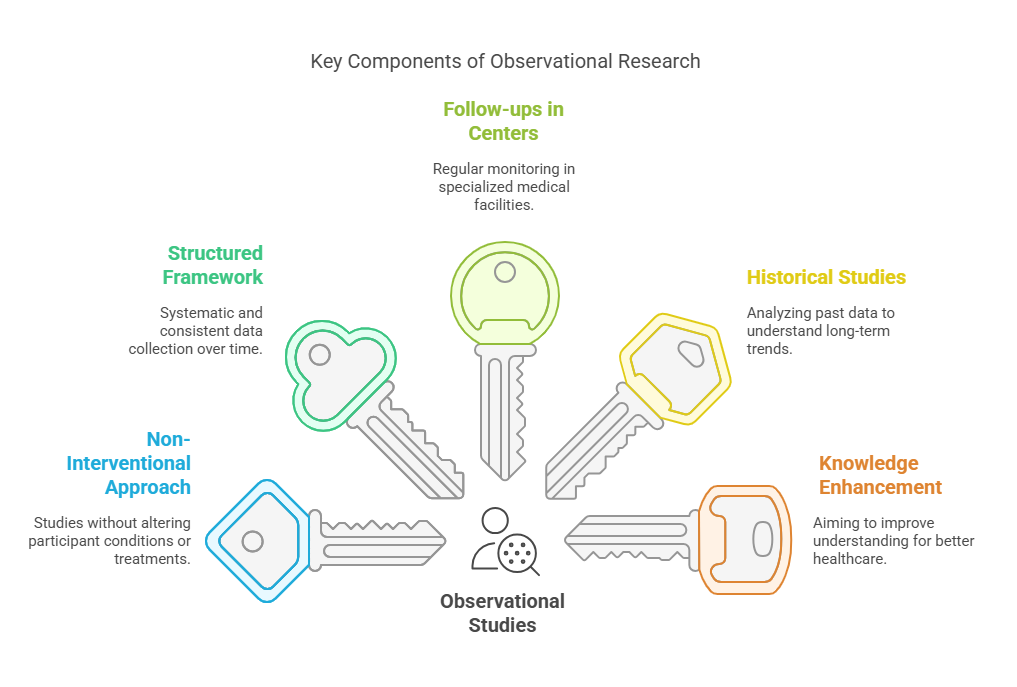
Observational studies are a fundamental component of clinical research focused on gaining a deeper understanding of diseases. These studies are primarily concerned with observing and recording the progression and various characteristics of a disease over time without manipulating the study environment or the subjects involved.
Key Characteristics of Observational Studies:
Non-Interventional Approach: Unlike clinical trials, observational studies do not involve interventions like administering new drugs or treatments. Instead, researchers observe participants under normal conditions to gather data on the natural progression of diseases.
Structured Framework: These studies are carried out within a well-defined framework which often involves systematic follow-ups with patients. This structured approach ensures consistency and reliability in the data collected, providing a clear timeline of disease progression and outcomes.
Follow-ups in Reference Centers: Participants are regularly monitored in specialized centers or hospitals, known as reference centers, which are equipped to provide detailed assessments and record comprehensive data on the disease’s evolution. These centers are pivotal in maintaining the continuity and precision of the observational studies.
Inclusion of Historical Studies: Observational research also includes retrospective analyses, where researchers study existing data from past cases (historical studies). This helps in understanding long-term trends and outcomes of diseases, thereby contributing to a broader knowledge base.
Objective of Knowledge Enhancement: The primary goal of these studies is to enhance understanding of a disease, focusing on aspects such as its natural history, risk factors, and long-term effects on patients. This improved knowledge is crucial for developing better preventive measures, diagnostic tools, and treatments in the future.
Clinical trials or Intervention studies
Clinical trials, also known as intervention studies, are critical components of clinical research aimed at assessing the safety and effectiveness of new medical treatments, including drugs, management strategies, and devices. These trials are meticulously designed to provide robust scientific evidence necessary for regulatory approval and subsequent market availability of these innovations.
Key Steps and Components of Clinical Trials:
Administration to Healthy Humans:
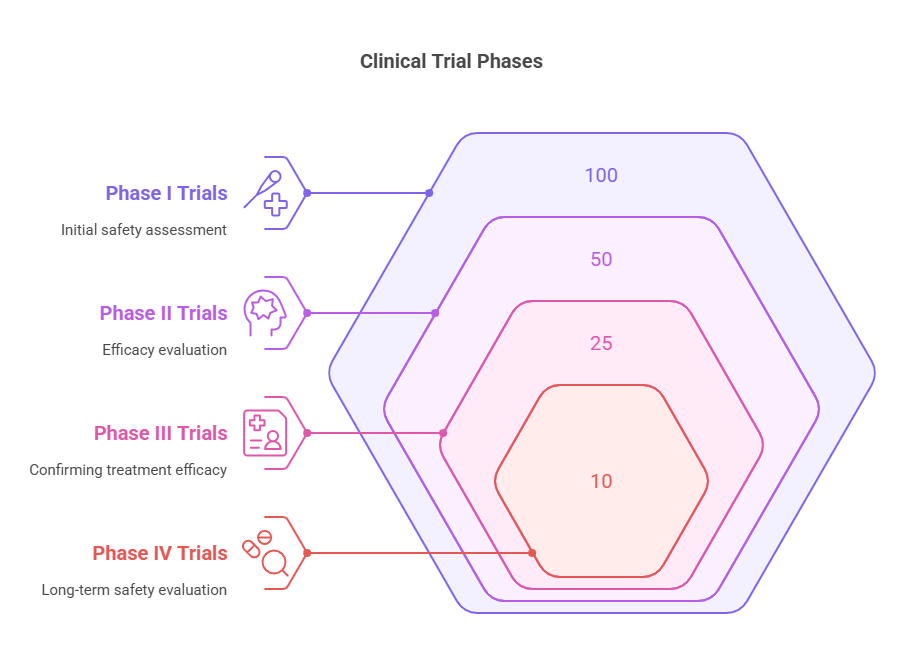
Phase I Trials: Often the first step in testing new treatments, where a small group of healthy volunteers is administered the drug or treatment to assess its safety, determine safe dosage ranges, and identify side effects. The primary focus here is to establish the treatment's safety profile rather than its efficacy.
Administration in Patients:
Phase II Trials: Once the initial safety of the treatment is established, it is administered to a larger group of patients who have the disease or condition that the treatment is aimed at. This phase aims to evaluate the treatment's efficacy and further assess its safety.
Therapeutic Efficacy:
Phase III Trials: This phase involves even larger groups of patients and is designed to confirm the treatment's efficacy, monitor side effects, compare it to commonly used treatments, and collect information that will allow the treatment to be used safely.
Marketing Authorization or Approval:
Once a treatment has successfully passed through the initial three phases, the data collected is submitted to regulatory authorities (such as the FDA in the United States) for review. This review process aims to ensure that the benefits of the drug or device outweigh its risks. If approved, the treatment receives marketing authorization, allowing it to be sold and prescribed.
Evaluation and Monitoring of Side Effects:
Phase IV Trials: Even after approval, the treatment's effects are monitored in a larger population and over a longer period to ensure continued safety and efficacy. This phase may uncover additional information about the treatment's risks, benefits, and optimal use, or long-term side effects that were not apparent in earlier phases.
Importance of Regulatory Compliance and Ethical Standards:
Clinical trials are highly regulated and must adhere to strict ethical standards to protect participants from undue harm. These standards include informed consent, rigorous oversight by ethical review boards, and adherence to scientific and ethical standards at every phase of the trial. This rigorous process ensures that by the time a new treatment reaches the general population, it has been thoroughly tested and its benefits and risks are well understood, thus safeguarding public health while fostering medical innovation.
Clinical trials
Clinical trials fall under the clinical research umbrella. It is an experiment which is designed to address very specific questions regarding possible treatments or any new ways that can be used with existing treatments. They are done to determine if the treatments and new drugs are effective and safe. Clinical trials are usually a long and careful process that can take years before completion. The treatment has to be studied in a lab by qualified doctors.
Drugs and treatments need to be animal tested before any human testing.
Medical care and clinical research
It is common for people to confuse medical care and clinical research. In Medicare, a patient has a plan that is unique to their needs. The treatment plans have already been tested and proven safe and effective.
Everyone who wants to undertake clinical trials should be aware that it is an experiment. This means that while patients will have access to the newest technology, clinical trial may or may not benefit you.
In clinical research, there is usually a pre-determined set plan or protocol that the researcher and the subject have to follow. In a clinical trial, there are steps taken if someone starts feeling unwell during the trials, but the researcher is not allowed to make any adjustments to the plan to fit any particular subject. It is therefore important to have everything laid out with the researcher and your doctor.
Conclusion
In conclusion, clinical research, including both observational studies and clinical trials, plays an indispensable role in advancing medical knowledge and enhancing patient care. Through rigorous protocols and a structured framework, these studies ensure the safety and efficacy of new treatments before they reach the market. Organizations like the Certified Clinical Research Professionals Society (CCRPS) provide essential training and certification to uphold the standards and integrity of this vital field. By supporting professionals in clinical research, CCRPS helps ensure that these complex studies are conducted ethically and effectively, leading to better health outcomes for all.
Explore Courses for Clinical Research Career
Courses Available:
Frequently Asked Questions (FAQs)
-
The main purpose of clinical research is to gather data on the safety and efficacy of new medical interventions, such as drugs, devices, and treatment strategies, to ensure they are safe and effective for human use before they are brought to the market. This research helps in improving and developing new methods for diagnosing, treating, and preventing diseases.
-
Clinical research focuses on studying health and illness in humans to develop knowledge that improves medical care, often through trials that test new treatments or procedures. Medical treatment, on the other hand, involves using established practices and interventions to manage individual patients' health based on current best practices and the patient's specific needs.
-
Clinical trials are typically divided into four phases: Phase I tests a new drug or treatment in a small group of people for the first time to evaluate its safety, determine a safe dosage range, and identify side effects. Phase II focuses on efficacy and further safety testing. Phase III confirms these findings in larger groups and compares the new treatment to existing treatments. Phase IV occurs after FDA approval and involves long-term monitoring of effects.
-
Participants in clinical trials can vary widely and include healthy volunteers or patients with the condition that the trial aims to treat. Eligibility is based on specific criteria that typically include factors like age, gender, the type and stage of a disease, previous treatment history, and other medical conditions, which help ensure the safety of participants and the clarity of results.
-
Participants in clinical trials can benefit from gaining access to new treatments before they are widely available and receiving expert medical care at leading health care facilities during the trial. However, there are risks involved, including side effects from the treatment or intervention, the possibility of ineffective treatment, and the rigorous demands of the trial protocol.