What Is A Clinical Research Coordinator
What is a clinical research coordinator?
A Clinical Research Coordinator (CRC) is a key professional in the medical research field, responsible for overseeing clinical trials and ensuring they comply with ethical and regulatory standards. CRCs play an important role in managing study protocols, recruiting and monitoring participants, collecting data, and coordinating with principal investigators, sponsors, and regulatory bodies. Their work is essential for the development of new drugs, treatments, and medical devices.
As the demand for innovative healthcare solutions continues to grow, so does the need for skilled CRCs. With increasing clinical trials worldwide, pharmaceutical companies, hospitals, and research institutions seek qualified professionals to ensure trials run smoothly and efficiently. CRCs not only contribute to groundbreaking medical advancements but also help maintain patient safety and data integrity. This career offers a promising future for those interested in clinical research, patient care, and the advancement of medical science.
Understanding The Role Of A Clinical Research Coordinator
A Clinical Research Coordinator (CRC) plays an important role in clinical trials, ensuring that studies are conducted smoothly, ethically, and according to regulations. They work under the supervision of a principal investigator (PI), who is the lead researcher, but the CRC is responsible for handling many day-to-day tasks that keep the trial running efficiently. One of the main duties of a CRC is patient recruitment. This includes finding and enrolling participants who fit the study's eligibility conditions, educating them about the trial procedure, and getting their written consent. The CRC ensures that participants understand the risks and benefits before they agree to take part.
During the trial, the CRC manages all parts of the study, including scheduling patient visits, monitoring their health, and collecting necessary data, such as blood samples or test results. They also handle important documents, ensure the trial follows ethical guidelines, and report any issues. A CRC collaborates closely with principal investigators, doctors, nurses, and research teams to make sure the study follows the correct procedures. They also communicate with pharmaceutical companies, regulatory agencies, and ethics boards to keep everything in compliance with legal and safety standards. Their work is essential in making sure new treatments are safe and effective before they are approved for overall use.
Key Responsibilities Of A Clinical Research Coordinator
A Clinical Research Coordinator (CRC) plays an important role in managing clinical trials by ensuring that studies are conducted safely, ethically, and according to regulations. Their responsibilities include working with patients, handling trial documentation, monitoring progress, and coordinating with regulatory bodies to ensure compliance with industry standards. Below are some of their key responsibilities:
Obtaining Informed Consent from Participants: Before a clinical trial begins, the CRC must inform potential participants about the study’s purpose, procedures, risks, and benefits. They explain everything in simple terms, answer any questions, and ensure that participants voluntarily agree to join by signing an informed consent form, which is a necessary ethical requirement.
Ensuring Compliance with Good Clinical Practice (GCP) and Ethical Guidelines: Clinical trials must follow strict regulations to protect patients and ensure reliable results. CRCs ensure that the trial follows Good Clinical Practice (GCP) guidelines, ethical standards, and legal regulations. This includes maintaining patient confidentiality, ensuring fairness in participant selection, and adhering to safety protocols.
Managing Trial Documentation and Regulatory Submissions: A CRC is responsible for maintaining accurate and up-to-date records of the study. This includes preparing and submitting important documents to regulatory authorities, keeping track of patient data, and ensuring all paperwork meets industry and legal requirements. Proper documentation is essential for the approval and credibility of clinical research.
Monitoring Patient Progress and Handling Adverse Event Reports: Throughout the trial, the CRC monitors participants to track their health and response to treatment. If a patient experiences side effects or unexpected issues, the CRC must document and report these adverse events to ensure patient safety. Quick action and proper reporting help in assessing the risks of the treatment.
Coordinating with Sponsors, Regulatory Bodies, and Institutional Review Boards (IRBs): Clinical trials involve multiple stakeholders, including sponsors (pharmaceutical companies or research institutions), regulatory bodies, and Institutional Review Boards (IRBs) that oversee the study’s ethical aspects. The CRC acts as the main point of contact, ensuring smooth communication, submitting reports, and addressing any concerns raised by these organizations.
Want to explore a rewarding career in healthcare? Don’t miss our latest blog: Clinical Research Coordinator Jobs: Key Responsibilities Explained for 2025—a must-read for aspiring professionals!
Skills Required For A CRC
A Clinical Research Coordinator (CRC) must possess a combination of technical knowledge and soft skills to manage clinical trials effectively. Their role involves handling multiple responsibilities, ensuring regulatory compliance, and collaborating with various professionals involved in the research process. Below are the essential skills required for a successful career as a CRC.
Analytical Thinking: CRCs must carefully analyze clinical trial data, identify discrepancies, and ensure that all results are accurate. They also need to interpret research protocols and regulations to maintain compliance. Strong analytical skills help in assessing study progress and ensuring that data is collected and reported correctly.
Multitasking: A CRC is responsible for handling multiple aspects of a clinical trial, including patient care, documentation, and regulatory compliance. Balancing these tasks efficiently is important for keeping trials on schedule and ensuring smooth operations without delays.
Interpersonal Skills: Since CRCs work closely with principal investigators, patients, research teams, and regulatory bodies, excellent communication and relationship-building skills are essential. They must explain complex medical information to patients in an understandable way and cooperate effectively with team members.
Problem-Solving: Unexpected challenges, such as patient dropouts, protocol changes, or data discrepancies, are common in clinical trials. A CRC must think critically to resolve issues while ensuring that the trial remains legal and continues smoothly without compromising data integrity or patient safety.
Qualifications Required For A CRC
A Clinical Research Coordinator (CRC) must have the right educational background and certifications to excel in the field. Employers typically look for candidates with degrees in healthcare-related fields and obtaining professional certifications can further enhance career opportunities. Below are the key qualifications required to become a successful CRC.
Educational Background
Most CRC jobs require a bachelor’s degree in fields such as life sciences, nursing, pharmacy, biology, or healthcare-related disciplines. These programs provide essential knowledge of medical terminology, research methods, and ethical considerations in clinical trials. Some advanced roles may prefer candidates with a master’s degree in clinical research, public health, or a related field, as higher education can open doors to leadership positions.
Certifications
Earning a Clinical Research Coordinator (CRC) certification is an important step toward building a successful career in clinical research. The Clinical Research Coordinator Certification Course offered by CCRPS provides comprehensive training to help you develop the skills needed to manage clinical trials, ensure regulatory compliance, and manage patient safety. This course covers essential topics such as Good Clinical Practice (GCP) guidelines, trial documentation, ethical considerations, and data management. With a self-paced learning format, it is designed for both beginners and experienced professionals looking to enhance their credentials and advance in the field.
Steps to Becoming a Clinical Research Coordinator
Becoming a Clinical Research Coordinator (CRC) requires a combination of education, hands-on experience, and industry-recognized certifications. Here’s a step-by-step guide to starting and growing your career as a Clinical Research Coordinator.
Earn a Relevant Degree: The first step to becoming a CRC is obtaining a bachelor’s degree in a relevant field such as life sciences, nursing, public health, biology, or healthcare administration. These fields provide the necessary foundation in medical terminology, research methodologies, and ethical guidelines. Some advanced positions may prefer candidates with a master’s degree in clinical research or healthcare management, which can offer deeper knowledge and open doors to higher-level roles.
Gain Experience in Healthcare or Research: Hands-on experience is essential for understanding the practical factors of clinical research. Aspiring CRCs should look for opportunities in hospitals, research labs, clinical research organizations (CROs), or pharmaceutical companies. Entry-level roles such as research assistants, data coordinators, or patient care technicians provide valuable exposure to clinical trial processes, regulatory guidelines, and patient interactions. This experience helps develop the skills necessary for managing studies efficiently.
Pursue Certifications: Obtaining professional certifications enhances a candidate’s credibility and career prospects. Two widely recognized certifications include the Certified Clinical Research Coordinator (CCRC) from the Association of Clinical Research Professionals (ACRP) and the Certified Clinical Research Professional (CCRP) from the Society of Clinical Research Associates (SOCRA). These certifications show expertise in regulatory compliance, ethical considerations, and clinical trial coordination. Many employers prefer or require these credentials, as they validate an individual’s knowledge in the field.
Develop Key Skills: A CRC must have essential skills to manage clinical trials effectively. Understanding guidelines helps follow Good Clinical Practice (GCP) and ethical research rules. Data management skills help in collecting, documenting, and analyzing trial data accurately. Patient care expertise is also important, as CRCs must ensure participants' safety, obtain informed consent, and maintain ethical treatment throughout the study. Developing these skills improves efficiency and enhances overall trial success.
Apply for Entry-Level CRC Positions: Once qualified, candidates should begin applying for CRC positions in hospitals, pharmaceutical companies, CROs, and academic research institutions. Entry-level roles provide practical experience in trial coordination, protocol implementation, and patient interaction. Working under experienced research professionals allows CRCs to gain confidence in managing studies and prepares them for advanced career opportunities in clinical research.
Continue Professional Development: To stay competitive and advance in their careers, CRCs should focus on continuous learning and professional growth. Attending workshops, industry conferences, and training programs helps them stay updated on evolving regulations and research processes. Obtaining advanced certifications and enrolling in a master’s program in clinical research or healthcare administration can further enhance career prospects. Professional development ensures CRCs remain knowledgeable and well-equipped to take on leadership roles in clinical research.
Want to learn how to become a Clinical Research Coordinator? Check out our detailed guide: 7 Steps to Becoming a Clinical Research Coordinator and explore everything you need to know to kick-start your career in clinical research!
Career Path and Growth Opportunities
A career as a Clinical Research Coordinator (CRC) offers diverse opportunities for growth in the healthcare and research industry. With experience and additional qualifications, professionals can progress to higher roles in clinical research and project management.
1. Entry-Level Roles and Becoming a CRC: Most aspiring CRCs start in entry-level positions such as clinical research assistants, research technicians, or data coordinators. These roles provide hands-on experience in managing clinical trials, handling patient data, and ensuring compliance with research protocols. By gaining relevant work experience in hospitals, pharmaceutical companies, or research institutions, candidates can transition into a Clinical Research Coordinator position. Obtaining industry-recognized certifications, such as the Certified Clinical Research Coordinator (CCRC) from ACRP or Certified Clinical Research Professional (CCRP) from SOCRA, further strengthens their qualifications and employability
2. Career Progression: From CRC to Advanced Roles: With experience and additional training, CRCs can advance to senior roles such as Clinical Research Associate (CRA), Lead CRC, or Clinical Project Manager. A CRA manages multiple clinical trials, ensuring regulatory compliance, site management, and protocol adherence. Those interested in leadership roles may transition to Clinical Trial Managers or Project Managers, where they handle large-scale research projects, supervise teams, and coordinate with sponsors and regulatory agencies. Pursuing a master’s degree in clinical research, healthcare administration, or business management can accelerate career growth and open doors to senior positions.
3. Job Opportunities in Various Sectors: CRCs have employment opportunities in a variety of settings, including:
Hospitals & Medical Centers – Managing clinical trials for new treatments and medical advancements.
Pharmaceutical & Biotechnology Companies – Conducting drug and vaccine trials for regulatory approval.
Contract Research Organizations (CROs) – Supporting research for multiple pharmaceutical and biotech firms.
Academic & Government Research Institutions – Leading public health and medical research initiatives.
4. Remote Clinical Research Coordinator Jobs: With advancements in technology, many CRCs now have the option to work remotely. Remote CRC Jobs involve tasks like data management, regulatory compliance, patient monitoring, and trial coordination, which can be done online. Some companies, especially CROs and pharmaceutical firms, offer fully remote or hybrid work opportunities, allowing professionals to contribute to clinical research from anywhere.
Salary and Earning Potential
The salary of a Clinical Research Coordinator (CRC) depends on factors like location, experience, and the type of employer. Hospitals, pharmaceutical companies, and research institutions may offer different pay scales based on their budgets and the complexity of the clinical trials they conduct.
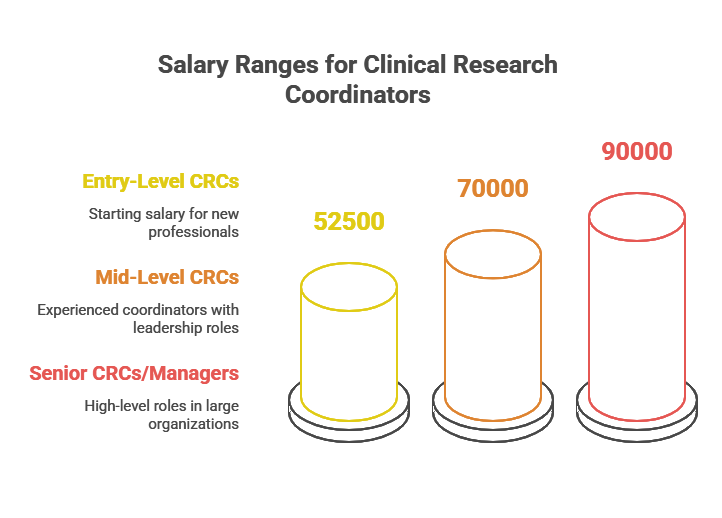
Entry-Level CRCs: Those starting in the field can expect to earn between $45,000 and $60,000 per year. At this stage, responsibilities typically include assisting with trial documentation, patient recruitment, and regulatory compliance under the supervision of senior staff.
Mid-Level CRCs (3–5 Years of Experience): With a few years of experience, CRCs can advance to roles that involve more responsibilities, such as leading clinical trial operations and managing study protocols. Salaries at this level typically range from $60,000 to $80,000 per year.
Senior CRCs or Managers: Experienced CRCs who move into senior or managerial positions, especially in large hospitals, pharmaceutical companies, or biotech firms, can earn over $90,000 per year. These roles may involve overseeing multiple trials, training junior staff, and ensuring compliance with complex regulations.
Wondering how much a Clinical Research Coordinator earns? Get the full breakdown of salaries at every career stage! From entry-level to senior roles by reading our complete blog on Clinical Research Coordinator Salary.
Challenges Faced By Clinical Research Coordinators
Clinical Research Coordinators (CRCs) play an important role in managing clinical trials, but their job comes with several challenges. To guarantee trial success, CRCs must remain organized, flexible, and precise while managing several trials concurrently and overcoming severe limitations. Below are some of the key challenges they face.
Managing Complex Regulatory Requirements: CRCs must stick to strict regulatory guidelines, including Good Clinical Practice (GCP), FDA regulations, and Institutional Review Board (IRB) protocols. Keeping up with evolving compliance standards can be challenging, as any deviation can lead to delays, legal issues, or trial termination. Ensuring proper documentation, ethical approvals, and data integrity is important to meeting these regulatory expectations.
Ensuring Participant Retention in Long-Term Trials: Many clinical trials require participants to stay engaged for months or even years. However, dropout rates can be high due to factors like inconvenient follow-ups, side effects, or lack of motivation. CRCs must develop strong communication skills and build trust with participants, ensuring they understand the trial’s importance and feel supported throughout the process. Offering flexible scheduling, reminders, and regular check-ins can help improve retention rates.
Balancing Multiple Studies Simultaneously: CRCs often manage multiple clinical trials at the same time, each with its own protocols, patient groups, and deadlines. It takes excellent time management and organizational abilities to balance many studies while maintaining accuracy in data collecting, compliance, and participant care. Prioritizing tasks, using clinical trial management software, and maintaining clear communication with research teams can help CRCs handle these demands efficiently.
Conclusion
Clinical Research Coordinators (CRCs) play an essential role in the success of clinical trials by ensuring that research is conducted ethically, efficiently, and in compliance with regulatory standards. Their work directly impacts the development of new treatments, medical advancements, and patient safety. For those interested in healthcare and research, a career as a CRC offers growth, job stability, and the opportunity to contribute to the medical field.
With the increasing demand for clinical research professionals, now is a great time to enter this field. If you’re considering a career as a Clinical Research Coordinator, start by exploring certification programs, gaining hands-on experience, or applying for entry-level positions in hospitals, research institutions, or pharmaceutical companies. Take the next step toward a rewarding career in clinical research today!