What is a Clinical Trial Specialist?
A clinical trial specialist (CTS) plays a significant role in the improvement of health care through research by monitoring clinical trials of new drugs, treatments, and medical devices. They guarantee that the trials are conducted appropriately, that is, they adhere to the laws, ethical practices and lead to accurate results.
As the scope of clinical research specialist job descriptions and the regulatory standards increase, the need is expected to for more skilled clinical trial specialists in 2025 and beyond. Whether you are seeking to start or grow your career, it helps to know what direction the job market is going, as well as the salary expectations and qualifications.
What Does a Clinical Trial Specialist Do?
A clinical trial specialist job description varies depending on the organization and phase of the trial. However, common responsibilities include:
Designing and writing study protocols
Ensuring compliance with regulatory requirements (FDA, EMA, ICH-GCP guidelines)
Managing clinical trial budgets and timelines
Collaborating with principal investigators, sponsors, and regulatory agencies
Ensuring accurate data collection and management
Training clinical research staff
Overseeing trial documentation for audits and inspections
In larger organizations, these professionals may work with clinical research regulatory specialists—experts who specialize in compliance with international regulatory guidelines, aside from them.
Why Clinical Trial Specialists Are in High Demand ?
The healthcare industry is experiencing rapid innovation, from clinical trials specialists conducting groundbreaking gene therapy studies to clinical study specialists managing precision medicine trials. Several factors are fueling the demand for experienced professionals:
1. Expansion of Clinical Trials
The global push for clinical trials specialists has increased due to:
The aging population and rise of chronic diseases
The development of advanced biologics and personalized medicine
Growing investments in AI-driven clinical research
2. Stricter Regulatory Compliance
With regulatory bodies tightening their oversight, companies require clinical research regulatory specialists to navigate the complexities of trial approvals, ethical guidelines, and patient safety protocols.
3. Technological Advancements in Clinical Trials
AI, blockchain, and big data analytics are transforming the way clinical trials are conducted. These advancements require specialists with experience in clinical trials regulatory compliance, data security, and decentralized trials.
How to Become a Clinical Research Specialist?
If you're wondering how to become a clinical research specialist, the path generally involves:
Step 1: Education
Most clinical research specialists hold a bachelor’s degree in life sciences, nursing, public health, or a related field. Advanced degrees (e.g., a master’s in clinical research) and certifications enhance career prospects.
Step 2: Gain Experience in Clinical Trials
Entry-level roles like clinical study specialist or trial specialist help professionals gain hands-on experience. Internships and research assistant positions also provide a solid foundation.
Step 3: Obtain Certifications
Certifications strengthen your expertise in regulatory requirements and clinical trial operations. Some recognized programs include:
Certified Clinical Research Professional (CCRP)
Clinical Research Coordinator (CRC) Certification
Clinical Trial Regulatory Specialist Certification
Step 4: Networking and Professional Growth
Attend industry conferences, join clinical research associations, and build connections on LinkedIn to stay ahead in this evolving field.
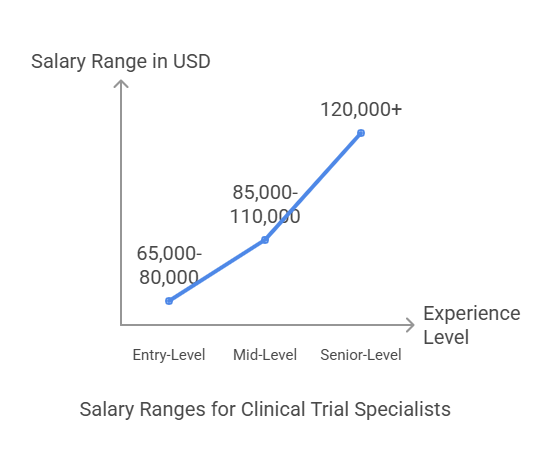
Clinical Trial Specialist Salary Expectations
A clinical trial specialist salary varies based on experience, location, and industry sector. In the U.S., the estimated salary ranges are:
Entry-Level (0-3 years experience): $65,000 - $80,000
Mid-Level (3-7 years experience): $85,000 - $110,000
Senior-Level (8+ years experience): $120,000+
Clinical research specialists working in biotech and pharmaceutical companies may receive higher salaries, especially those with regulatory expertise.
Career Growth and Opportunities for Clinical Trial Specialists
A career in clinical research offers long-term growth opportunities. Professionals can transition into roles such as:
Clinical Research Manager – Overseeing multiple trials and research teams
Clinical Trial Regulatory Specialist – Focusing on compliance and global regulatory approvals
Clinical Research Director – Leading strategic trial planning and execution
Conclusion
A career in clinical research specialist jobs provides stability, growth, and the opportunity to contribute to life-saving medical discoveries. If you’re detail-oriented, passionate about research, and eager to work in a dynamic field, becoming a clinical trial specialist in 2025 could be an excellent choice.
With the continued rise in clinical trials, regulatory demands, and technological advancements, now is the perfect time to step into this rewarding career path. CCRPS offers accredited training programs to help you gain the skills and certifications needed to succeed in this evolving industry.
Reference Links:
Johns Hopkins University - Clinical Research Training and Education
National Institutes of Health (NIH) - Careers in Clinical Research
U.S. Food and Drug Administration (FDA) - Clinical Trials and Regulatory Compliance
European Medicines Agency (EMA) - Clinical Trials Regulations
World Health Organization (WHO) - Clinical Trials and Good Clinical Practice Guidelines
Explore Courses for Clinical Research Career
Courses Available: