What Is Good Clinical Practice (GCP)
What is good clinical practice (GCP)?
Clinical trials play a major role in developing new treatments, but they must be conducted in a way that keeps participants safe and ensures accurate results. To maintain fairness in research, strict guidelines are followed worldwide. These guidelines help researchers, doctors, and companies test new medicines properly while protecting the people involved. Without proper rules, clinical trials could be unsafe, and the findings might not be reliable.
There are many steps involved in keeping research fair and ethical. Participants must give their permission before joining a trial, and their personal information must be kept private. Researchers are also required to report results truthfully and follow strict procedures to avoid mistakes or preferences. Regulatory organizations, such as the Food and Drug Administration (FDA) in the United States, make sure these rules are followed. While different countries have their own guidelines, they all share the same goal of ensuring precision and safety in research.
Following strict research standards helps build trust in medical advancements. When scientists follow these guidelines, they can confirm that new treatments are safe before they reach the public. This reduces the risk of harmful effects and ensures that only effective medicines are approved. People who join clinical trials can feel confident that their health and rights are protected. In the long run, strong research practices help improve healthcare by ensuring that every new treatment is well-tested and reliable.
What Is Good Clinical Practice (GCP)?
Medical research requires strong guidelines to ensure that clinical trials are reliable and safe. Good Clinical Practice (GCP) provides a structured system that helps researchers conduct studies with accuracy and transparency. It ensures that trials follow ethical rules, protecting the rights of participants while also maintaining the quality of collected data. These guidelines not only build trust in new treatments but also help doctors and scientists make informed decisions about the effectiveness of medicines.
Another important part of GCP compliance is the need for proper training of research teams. Everyone involved in a clinical trial, from doctors to data collectors, must understand and follow Good Clinical Practice guidelines to avoid mistakes and ensure smooth trial operations. This training helps reduce errors, ensures accurate reporting, and maintains patient safety throughout the study. By following these principles, clinical trials can provide results that benefit both the medical field and public health.
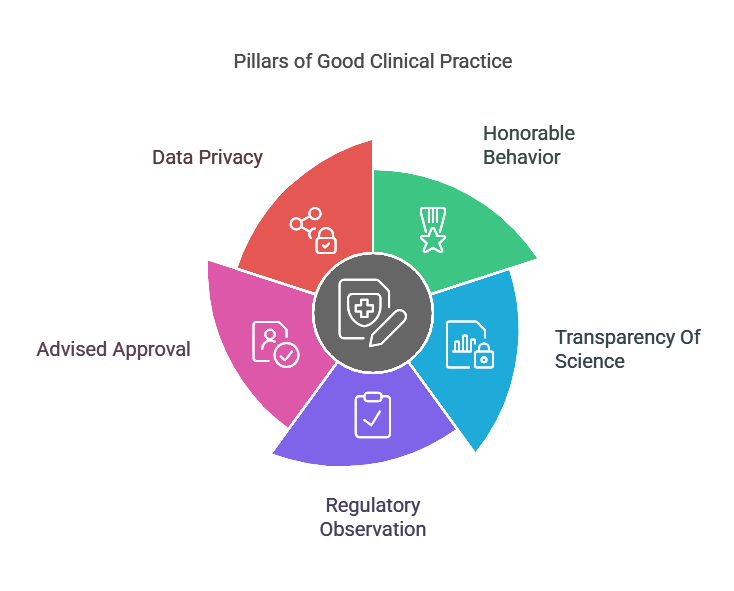
Essential Pillars Of Good Clinical Practice
Good Clinical Practice (GCP) is built on key principles that help maintain fairness, accuracy, and safety in clinical trials. These principles provide that trials are carried out in a responsible way, protecting both the participants and the reliability of research data. By following GCP compliance rules, researchers can prevent mistakes, safeguard patient rights, and provide reliable results. Below are the essential pillars that support Good Clinical Practice guidelines and help maintain high standards in medical research.
1. Honorable Behavior
A clinical trial must always prioritize the well-being of participants. Honorable behavior means that every study is planned and conducted in a way that protects human dignity and prevents harm. Researchers must be honest about potential risks and benefits while ensuring that trials are fair. Good Clinical Practice guidelines require that all medical studies follow strict ethical standards to avoid exploitation and maintain public trust. By upholding ethical conduct, research teams create a safe environment where participants are treated with care and respect.
2. Transparency Of Science
The success of medical research depends on the accuracy of the data collected. Good Clinical Practice ensures that studies follow structured methods to avoid errors, bias, or false reporting. Every result must be recorded honestly, without any influence from outside interests. GCP compliance also requires that researchers use reliable techniques to analyze data, ensuring that findings are meaningful and useful. Without scientific integrity, trial results could be misleading, which may put patients at risk and slow down medical progress.
3. Regulatory Observation
Clinical trials must meet national and international standards to be considered valid. GCP compliance ensures that researchers follow all legal requirements set by health management. These rules help protect patients while also ensuring that trial results can be trusted by doctors and regulatory tools. Good Clinical Practice guidelines require ongoing monitoring of trials to confirm that they meet necessary safety and ethical standards. Without regulatory compliance, new treatments may not receive approval, delaying their availability to patients in need.
4. Advised Approval
Every person who joins a clinical trial must do so willingly, with a full understanding of the risks and benefits involved. Good Clinical Practice guidelines require researchers to provide clear, honest information about the study before participants agree to take part. Informed consent means that no one is forced or misled into joining a trial. It also gives participants the right to leave the study at any time without pressure. By respecting autonomy, GCP compliance helps build trust and ensures that medical research remains ethical and voluntary.
5. Data Privacy
Clinical trials involve sensitive personal information, and it is important to keep this data private. Good Clinical Practice ensures that participant records are securely stored and only shared with authorized personnel. Researchers must take strong measures to prevent data leaks or misuse. GCP compliance also requires that patient identities are protected, ensuring that personal health details remain confidential. When data privacy is maintained, participants feel safer about joining clinical trials, which encourages more people to contribute to medical advancements.
The Importance Of Good Clinical Practice (GCP)
Clinical trials play an essential role in developing new treatments, but they must be conducted safely and fairly. Good Clinical Practice (GCP) ensures that research follows strict guidelines to protect participants and produce reliable results. Without these guidelines, clinical studies could be unsafe, and their findings might not be trustworthy. GCP compliance helps maintain high research standards, making sure that medical advancements benefit patients while keeping ethical and scientific integrity intact.
Protects Human Participants
One of the main purposes of Good Clinical Practice guidelines is to keep participants safe. Before any trial begins, researchers must explain the potential risks and benefits so that participants can make informed decisions. GCP compliance also ensures that strict monitoring takes place throughout the study to prevent harm. If a treatment shows dangerous side effects, trials can be adjusted or stopped immediately. By putting participant safety first, GCP helps create ethical and responsible research.
Ensures Data Accuracy And Reliability
For clinical research to be useful, its results must be precise and truthful. Good Clinical Practice requires researchers to follow structured methods when collecting and analyzing data. This reduces errors, prevents bias, and ensures that the findings are meaningful. GCP compliance also requires proper documentation, so research can be reviewed and verified by regulatory agencies. Without accurate data, medical treatments might be ineffective or even harmful, delaying progress in healthcare.
Builds Public Trust In Clinical Research
People are more likely to support and participate in clinical trials when they trust the research process. Good Clinical Practice guidelines help build this trust by ensuring fairness, transparency, and accountability. When participants know their safety is a priority and that research is conducted honestly, they feel more confident in contributing to medical advancements. GCP compliance also reassures doctors, healthcare organizations, and the public that new treatments are tested thoroughly before approval.
Understanding GCP Guidelines And Regulatory Bodies
Good Clinical Practice (GCP) follows a set of rules that help make clinical research safe and reliable. The International Council for Harmonisation (ICH-GCP) created these rules to ensure that clinical trials are done the same way across different countries. These Good Clinical Practice guidelines focus on protecting participants, keeping research fair, and making sure the results are accurate. Many countries follow these guidelines to maintain high standards in medical research. GCP compliance is important because it helps researchers avoid errors and ensures that new treatments are tested properly before they are approved for public use.
In the United States, the Food and Drug Administration (FDA) is responsible for making sure clinical trials follow Good Clinical Practice guidelines. The FDA checks that researchers follow strict procedures, keep patient data private, and report results honestly. If a study does not meet GCP compliance, the FDA can stop the trial or reject its findings. Other countries have similar organizations that enforce these rules to keep clinical research safe and effective. By following Good Clinical Practice, medical advancements can be made while keeping patient safety a top priority.
How To Maintain GCP Compliance In Research
Maintaining GCP compliance in research requires careful planning and strict adherence to rules. Investigators and sponsors must follow Good Clinical Practice guidelines to ensure trials are safe and reliable. They should start by developing a clear research plan, ensuring that all steps follow ethical and scientific standards. Proper training for staff is also important so that everyone understands their responsibilities. Keeping detailed and accurate records is another key step, as this helps regulatory bodies review the trial. Regular monitoring and audits can identify mistakes early and prevent issues that may affect the study’s validity.
Despite careful planning, researchers often face challenges while maintaining GCP compliance. One common problem is improper documentation, which can lead to missing or inaccurate data. To fix this, teams should create a system for organizing and reviewing records regularly. Another challenge is ensuring that participants fully understand the study before giving consent. Clear communication and detailed explanations help in overcoming this issue. Additionally, keeping up with regulatory updates can be difficult, so researchers should stay informed about any changes in Good Clinical Practice guidelines to avoid mistakes.
Conclusion
Looking to advance your career in clinical research? The Certified Clinical Research Professionals Society (CCRPS) provides top-tier training programs designed to help you achieve GCP compliance and excel in the industry. With expert-led courses covering Good Clinical Practice (GCP) guidelines, clinical trial management, and regulatory compliance, CCRPS ensures you gain the skills needed for success. Trusted by professionals in 81 countries and over 1,576 hiring organizations, our programs open doors to exciting opportunities in the field. Start your journey today by getting in touch with us by sending us an email at support@ccrps.org or giving us a call at +1 (239) 329-9837. Join thousands of professionals who trust CCRPS for career growth in clinical research!