Clinical Trial Monitoring Reports and How to Write Them
Among the aspects in study is observation. Overseeing the advancement of any stage, measure, process, and procedure in real time is essential to the accurate conclusion of any clinical trial undertaking. Normal monitoring actions are needed to guarantee caliber , efficiency, compliance with predefined and regulations fundamentals, in addition to comprehensiveness, and precision within clinical investigation. Such actions also ensure that the trial isn't just conducted in compliance with Standard Operating Procedures (SOPs) however they also function to validate it is correctly reported and recorded. There is something with a part in the execution of trials. And this thing is known as trial development reports.
Such a report ought to be carefully prepared and it must summarize the means that a study is done. It also ought to point out recruiting progress and procedures; should emphasize and clarify adjustments to the analysis, and ought to point security issues if there are not some.
Aside from the ethics committee, researchers could also be necessary to present yearly improvement reports of an investigation (such as any applicable alterations or dangers ) to spouses, encouraging associations, and/or organizations, along with other interested parties if needed.
If you're thinking about getting important skills on GCP or you also would like to upgrade your own know-how, subscribe to our comprehensive Great Clinical Practice class here.
In summary, tracking and reporting processes have an incredibly significant function in clinical trials. The right behaviour of these procedures not merely ensures compliance with legislation, regulations, and predetermined conditions, but in addition, it makes certain the study doesn't pose any dangers to their wellbeing. Progress reports, subsequently, empower practitioners, specialists, researchers, ethics committees, as well as others involved to keep a tab on the trial and its own advancement. They may signal any alterations or risks and may, therefore, react to them timely, correctly, and efficiently.
The objective of progress reports would be to accumulate and outline upgrades, key facets, along with summaries of a continuing trial. It's very crucial to be aware that progress reports must be filed to institutional evaluation board/independent integrity questionnaire (IRB/IEC), following a trial has obtained positive opinion.
One other important issue to mention is there are many different forms as soon as it comes to submitting progress reports that researchers must take into consideration before proceeding. Precisely, these kinds are:
Printing name and date of entry ought to be composed also. A digital copy is also needed to be delivered to the interested websites and committees inside a 30-day interval following the reporting procedure was completed.
The period of time whereby an advance report ought to be filed is at least one time in a year. Nevertheless, based upon the situation as well as the RECs' needs, these reports might be passed in more often, although the analysis is still going and till its official conclusion date.
Among the most important aspects of study is observation. Overseeing the advancement of any stage, measure, process, and procedure in real time is essential to the accurate conclusion of any clinical trial undertaking. Normal monitoring actions are needed to guarantee caliber, efficiency, compliance within predefined and regulations fundamentals. In addition, it guarantees comprehensiveness, and precision within clinical investigation. Such actions also ensure that the trial isn't just conducted in compliance with Standard Operating Procedures (SOPs). They must also function to validate it is correctly reported and recorded. There demands something vital as a part in the execution of trials. And this thing is known as trial development reports.
Such a report ought to be carefully prepared and it must summarize the means that a study is done. It also ought to point out recruiting progress and procedures; should emphasize and clarify adjustments to the analysis, and ought to point security issues.
Aside from the ethics committee, researchers could also need to present yearly improvement reports of an investigation (such as any applicable alterations or dangers) to spouses, encouraging associations, and/or organizations, along with other interested parties if needed.
If you're thinking about getting important skills on GCP or you also would like to upgrade your own know-how, subscribe to our comprehensive Good Clinical Practice class here.
In summary, tracking and reporting processes is an incredibly significant function in clinical trials. The right conduct of these procedures ensure compliance with legislation, regulations, and predetermined conditions. In addition, they make certain that the study doesn't pose any dangers to wellbeing. Progress reports, subsequently, empower practitioners, specialists, researchers, ethics committees, as well as others involved to keep a tab on the trial and its own advancement. Clinical professionals need to signal any alterations or risks to react to them timely, correctly, and efficiently.
The objective of progress reports would be to accumulate and outline upgrades, key facets, along with summaries of a continuing trial. It's very crucial to be aware that progress reports must be filed to institutional evaluation board/independent integrity questionnaire (IRB/IEC), following a trial that has obtained positive opinion.
One other important issue to mention is there are many different forms and when it comes to submitting progress reports that researchers must take into consideration before proceeding. Precisely, these kinds are:
Printing name and date of entry ought to be composed also. A digital copy is also needed to be delivered to the interested websites and committees inside a 30-day interval following the reporting procedure was completed.
The period of time whereby an advance report ought to be filed is at least one time in a year. Nevertheless, based upon the situation as well as the RECs' needs, these reports might be passed in more often, although the analysis is still going and till its official conclusion date.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
2018 Clinical Research Associate (CRA) Salaries Estimated from 1,850 American Employees
Skills Alliance Clinical Research Associate
Clinical Research Associate Job available
$113,368 per year
Advanced Clinical Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$107,750 per year
Syneos Health Commercial Solutions Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$98,894 per year
Piper Companies Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$101,820 per year
Novella Clinical Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$97,659 per year
Covance Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$96,667 per year
Premier Research Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$95,178 per year
Syneos Health Clinical Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$93,469 per year
PRA Health Sciences Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$76,150 per year
Novo Nordisk, Inc. Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$95,242 per year
PPD Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$91,293 per year
IQVIA Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$89,615 per year
Cyberonics Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$87,000 per year
AbbVie Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$81,391 per year
ICON plc Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$79,110 per year
PAREXEL Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$81,281 per year
QuintilesIMS Clinical Research Associate
11 salaries
Clinical Research Associate Job available
$72,749 per year
MD Anderson Cancer Center Clinical Research Associate
7 salaries
Clinical Research Associate Job available
$71,410 per year
Chiltern International Clinical Research Associate
5 salaries
$87,722 per year
Skills Alliance Clinical Research Associate
Clinical Research Associate Job available
$113,368 per year
Advanced Clinical Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$107,750 per year
Syneos Health Commercial Solutions Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$98,894 per year
Piper Companies Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$101,820 per year
Novella Clinical Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$97,659 per year
Covance Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$96,667 per year
Premier Research Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$95,178 per year
Syneos Health Clinical Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$93,469 per year
PRA Health Sciences Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$76,150 per year
Novo Nordisk, Inc. Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$95,242 per year
PPD Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$91,293 per year
IQVIA Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$89,615 per year
Cyberonics Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$87,000 per year
AbbVie Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$81,391 per year
ICON plc Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$79,110 per year
PAREXEL Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$81,281 per year
QuintilesIMS Clinical Research Associate
11 salaries
Clinical Research Associate Job available
$72,749 per year
MD Anderson Cancer Center Clinical Research Associate
7 salaries
Clinical Research Associate Job available
$71,410 per year
Chiltern International Clinical Research Associate
5 salaries
$87,722 per year
Pharmanet Clinical Research Associate
5 salaries
$72,488 per year
Roche Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$71,586 per year
University of Florida Clinical Research Associate
11 salaries
Clinical Research Associate Job available
$69,492 per year
Department of Veterans Affairs Clinical Research Associate
6 salaries
Clinical Research Associate Job available
$66,274 per year
Abbott Laboratories Clinical Research Associate
6 salaries
Clinical Research Associate Job available
$66,095 per year
University of Utah Clinical Research Associate
19 salaries
Clinical Research Associate Job available
$66,775 per year
University of Minnesota Clinical Research Associate
8 salaries
Clinical Research Associate Job available
$64,600 per year
University of Connecticut Health Center Clinical Research Associate
8 salaries
Clinical Research Associate Job available
$58,321 per year
St. Jude Children's Research Hospital Clinical Research Associate
6 salaries
Clinical Research Associate Job available
$56,315 per year
Northwestern University Clinical Research Associate
6 salaries
Clinical Research Associate Job available
$54,342 per year
Duke Clinical Research Institute Clinical Research Associate
5 salaries
$73,447 per year
Emory University Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$50,778 per year
Medpace Clinical Research Associate
6 salaries
Clinical Research Associate Job available
$50,958 per year
University of South Florida Clinical Research Associate
20 salaries
Clinical Research Associate Job available
$50,810 per year
The University of Iowa Clinical Research Associate
26 salaries
Clinical Research Associate Job available
$43,547 per year
Yale University Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$40,378 per year
University of Maryland, Baltimore Clinical Research Associate
7 salaries
Clinical Research Associate Job available
$39,818 per year
University of North Carolina at Chapel Hill Clinical Research Associate
7 salaries
Clinical Research Associate Job available
$38,402 per year
University of North Carolina at Greensboro Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$38,475 per year
Virginia Commonwealth University Clinical Research Associate
19 salaries
Clinical Research Associate Job available
$36,453 per year
University of North Carolina Clinical Research Associate
15 salaries
$36,955 per year
University of Pittsburgh Clinical Research Associate
13 salaries
Clinical Research Associate Job available
$33,878 per year
SUNY Downstate Medical Center Clinical Research Associate
9 salaries
Clinical Research Associate Job available
$33,343 per year
Washington University in St. Louis Clinical Research Associate
18 salaries
Clinical Research Associate Job available
$15.75 per hour
UC San Diego Clinical Research Associate
19 salaries
Clinical Research Associate Job available
$4,353 per month
University of California - San Francisco Clinical Research Associate
6 salaries
Clinical Research Associate Job available
$100 per day
Real Staffing Clinical Research Associate
15 salaries
Clinical Research Associate Job available
$31.95 per hour
University of Nebraska Medical Center Clinical Research Associate
12 salaries
Clinical Research Associate Job available
$15.81 per hour
Texas Tech University Health Sciences Center Clinical Research Associate
6 salaries
Clinical Research Associate Job available
$3,609 per month
Aerotek Clinical Research Associate
8 salaries
$47.68 per hour
Randstad Clinical Research Associate
35 salaries
$45.90 per hour
Ohio State University Clinical Research Associate
124 salaries
Clinical Research Associate Job available
$14.49 per hour
University of Kentucky Clinical Research Associate
59 salaries
Clinical Research Associate Job available
$21.63 per hour
Biotech Partners Clinical Research Associate
12 salaries
Clinical Research Associate Job available
$111,431 per year
DOCS Global Clinical Research Associate
14 salaries
Clinical Research Associate Job available
$90,856 per year
CyberCoders Clinical Research Associate
28 salaries
$88,409 per year
Zp group Clinical Research Associate
18 salaries
$98,008 per year
CIG Clinical Research Associate
109 salaries
$116,784 per year
CorTech, LLC Clinical Research Associate
40 salaries
$38.91 per hour
Skills Alliance (Recruiting Company) Clinical Research Associate
12 salaries
$71.01 per hour
InfoStaff Clinical Research Associate
52 salaries
$109,699 per year
Stony Brook University Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$39,254 per year
Brio Resource Group Clinical Research Associate
46 salaries
Clinical Research Associate Job available
$129,987 per year
Planet Pharma Clinical Research Associate
7 salaries
Clinical Research Associate Job available
$117,458 per year
biorasi LLC Clinical Research Associate
18 salaries
$63,702 per year
Intermountain Home Care Clinical Research Associate
11 salaries
$15.55 per hour
Piper Clinical Solutions Clinical Research Associate
16 salaries
$64,853 per year
Personify Clinical Research Associate
36 salaries
$99,373 per year
Segal Trials Clinical Research Associate
24 salaries
$2,195 per month
Bertram & Associates Clinical Research Associate
7 salaries
$116,417 per year
Lodestar Executive Search Clinical Research Associate
7 salaries
$116,417 per year
PSU Personnel Services Unlimited, Inc. Clinical Research Associate
6 salaries
$104,485 per year
The Hastings Group Clinical Research Associate
7 salaries
$116,417 per year
Ploeger Recruiting Services Clinical Research Associate
6 salaries
$104,485 per year
Phillips Staffing Solutions Clinical Research Associate
6 salaries
$104,485 per year
PMG Employment Consultants Clinical Research Associate
7 salaries
$116,417 per year
Bio-Partners Clinical Research Associate
7 salaries
$116,417 per year
Hire Horizons Clinical Research Associate
6 salaries
$104,485 per year
SearchStars Clinical Research Associate
6 salaries
$104,485 per year
Fowler Placement Services, Inc. Clinical Research Associate
6 salaries
$104,485 per year
Plastic Executive Recruiters Clinical Research Associate
6 salaries
$104,485 per year
EPM Scientific Clinical Research Associate
13 salaries
Clinical Research Associate Job available
$98,268 per year
Barrington James Clinical Research Associate
12 salaries
Clinical Research Associate Job available
$124,820 per year
MedExec International Clinical Research Associate
6 salaries
$104,485 per year
Tri-Force Clinical Research Associate
7 salaries
$116,417 per year
Professional Recruiting Partners, LLC Clinical Research Associate
7 salaries
$116,417 per year
Career Brokers, Inc. Clinical Research Associate
6 salaries
$104,485 per year
Helffrich International Clinical Research Associate
6 salaries
$104,485 per year
Tailored Management Clinical Research Associate
10 salaries
$79,138 per year
MJ Recruiters, LLC Clinical Research Associate
6 salaries
$104,485 per year
Meet Recruitment Clinical Research Associate
12 salaries
$102,820 per year
University of Virginia Clinical Research Associate
16 salaries
Clinical Research Associate Job available
$44,455 per year
Medical University of South Carolina Clinical Research Associate
20 salaries
Clinical Research Associate Job available
$32,233 per year
Oregon Health Sciences University Clinical Research Associate
17 salaries
Clinical Research Associate Job available
$43,176 per year
AutoPro Technical Recruiting Clinical Research Associate
6 salaries
$104,485 per year
MMS Group Clinical Research Associate
6 salaries
$104,485 per year
ONESource Technical Clinical Research Associate
6 salaries
$104,485 per year
Technology Recruiting Solutions, Inc. Clinical Research Associate
6 salaries
$104,485 per year
BRUNEL CANADA Clinical Research Associate
20 salaries
$83,573 per year
Affinity Executive Search Clinical Research Associate
20 salaries
$111,284 per year
PPD Development, L.P. Clinical Research Associate
11 salaries
$92,737 per year
HireNetworks Clinical Research Associate
12 salaries
Clinical Research Associate Job available
$94,478 per year
CMC Limited Clinical Research Associate
13 salaries
$111,719 per year
CMD And Associates Clinical Research Associate
17 salaries
$97,982 per year
fulltimeGiGS Clinical Research Associate
10 salaries
$120,496 per year
Cameron Craig Group Clinical Research Associate
17 salaries
$88,715 per year
Prevalent Group Clinical Research Associate
17 salaries
$95,522 per year
True North Consultants Clinical Research Associate
6 salaries
$104,485 per year
Financially Stable Pharmaceutical Clinical Research Associate
8 salaries
$112,472 per year
JDP Search Group Clinical Research Associate
16 salaries
$99,326 per year
PeopleStaff Clinical Research Associate
15 salaries
$84,270 per year
Clinical Management Consultants Clinical Research Associate
9 salaries
Clinical Research Associate Job available
$115,881 per year
JOHNLEONARD Clinical Research Associate
8 salaries
Clinical Research Associate Job available
$61,509 per year
Rangam Consultants Inc. Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$72,617 per year
Keshav Consulting Solutions Clinical Research Associate
13 salaries
$60,670 per year
Onboard.jobs Clinical Research Associate
15 salaries
$94,156 per year
Cor-Tech LLC Clinical Research Associate
15 salaries
$42.97 per hour
Klinexa Inc. Clinical Research Associate
8 salaries
$25.00 per hour
Systematic business consulting Clinical Research Associate
13 salaries
$88,299 per year
University of Miami Miller School of Med Clinical Research Associate
8 salaries
$74,809 per year
Global Channel Management, Inc. Clinical Research Associate
12 salaries
$41.26 per hour
Productive Data Solutions, INC. Clinical Research Associate
8 salaries
Clinical Research Associate Job available
$27.00 per hour
Selective Staffing Solutions Clinical Research Associate
8 salaries
Clinical Research Associate Job available
$19.49 per hour
Atlanta Center for Medical Research Clinical Research Associate
5 salaries
$33,642 per year
DOCS Clinical Research Associate
10 salaries
$78.22 per hour
Impact Business Group Clinical Research Associate
12 salaries
$54.45 per hour
CPS Recruitment Clinical Research Associate
7 salaries
$28.46 per hour
SCOPE International USA, Inc. Clinical Research Associate
11 salaries
$80,886 per year
Interview with Center for Advanced Obstetrical Care and Research Clinical Research Associate
8 salaries
$17.00 per hour
Inflamax Research Inc. Clinical Research Associate
6 salaries
$2,900 per month
Priority Sales Recruiting Clinical Research Associate
11 salaries
$108,226 per year
University of Texas Medical Branch Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$16.50 per hour
Eastern Virginia Medical School Clinical Research Associate
10 salaries
Clinical Research Associate Job available
$82,730 per year
Computech Corporation Clinical Research Associate
7 salaries
$45.00 per hour
Details on application Clinical Research Associate
9 salaries
$205,058 per year
BK Rich Associates Clinical Research Associate
6 salaries
$90,000 per year
Central Florida Careers Clinical Research Associate
9 salaries
$16.93 per hour
Chipton Ross Clinical Research Associate
10 salaries
$34.37 per hour
AppleOne Clinical Research Associate
10 salaries
$75,534 per year
Sabio Systems Clinical Research Associate
9 salaries
$19.90 per hour
Blair Search Clinical Research Associate
7 salaries
$116,417 per year
Sponsor Pharmaceutical company Clinical Research Associate
9 salaries
$110,000 per year
Helix Biomedics LLC Clinical Research Associate
6 salaries
$12.00 per hour
Ability Professional Network Clinical Research Associate
9 salaries
$95,663 per year
Leading CRO Company!! Clinical Research Associate
5 salaries
$99,504 per year
The Pursell Group Clinical Research Associate
7 salaries
$116,417 per year
PeaceHealth Clinical Research Associate
9 salaries
$35.36 per hour
new england physician recruitment center-boston Clinical Research Associate
9 salaries
$180,000 per year
MBA IT Consulting Services, Inc. Clinical Research Associate
7 salaries
$116,417 per year
Food Management Search Clinical Research Associate
7 salaries
$116,417 per year
Pharmaceutical Organization Clinical Research Associate
8 salaries
$246,884 per year
Texas Tech University Health Sciences Center of El Paso Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$15.64 per hour
University of Wisconsin–Madison Clinical Research Associate
8 salaries
Clinical Research Associate Job available
$40,257 per year
Careers 2005 Clinical Research Associate
6 salaries
$104,485 per year
Taylee Staffing Clinical Research Associate
8 salaries
$102,227 per year
Rayco Technical Solutions, LLC Clinical Research Associate
6 salaries
$104,485 per year
Nishom Pharma Corp Clinical Research Associate
6 salaries
$70,000 per year
5 salaries
$57,250 per year
Real Life Sciences Clinical Research Associate
7 salaries
$117,035 per year
Inflamax Research Clinical Research Associate
6 salaries
$2,900 per month
Aptude Inc. Clinical Research Associate
7 salaries
$20.00 per hour
Virginia Commonwealth University Clinical Research Associate
8 salaries
Clinical Research Associate Job available
$36,729 per year
Scientific Search Clinical Research Associate
7 salaries
Clinical Research Associate Job available
$85,322 per year
ER Squared, Inc. Clinical Research Associate
6 salaries
$50,000 per year
Valesta Clinical Research Solutions Clinical Research Associate
7 salaries
$65,000 per year
On-Board Services Clinical Research Associate
5 salaries
$38.67 per hour
Military4Hire Clinical Research Associate
6 salaries
$104,485 per year
SSC Clinical Research Associate
8 salaries
$115,000 per year
Enterprise Search Associates Clinical Research Associate
6 salaries
$104,485 per year
The Fountain Group Clinical Research Associate
8 salaries
$135,000 per year
VetPharm, Inc. Clinical Research Associate
8 salaries
$47,500 per year
Pioneer Data Systems, Inc. Clinical Research Associate
7 salaries
Clinical Research Associate Job available
$50.00 per hour
The University of Michigan Clinical Research Associate
6 salaries
Clinical Research Associate Job available
$60,183 per year
North Peak Recruiting Clinical Research Associate
6 salaries
$104,485 per year
MedicusTek USA, Inc Clinical Research Associate
7 salaries
$42,000 per year
Genesis Global Management Corporation Clinical Research Associate
7 salaries
$105,971 per year
Quality Clincial Research Clinical Research Associate
5 salaries
$28,802 per year
The Job Jobber Clinical Research Associate
7 salaries
$88,043 per year
IMARC Research, Inc. Clinical Research Associate
5 salaries
$15.52 per hour
PHAIDON INTERNATIONAL Clinical Research Associate
6 salaries
$109,198 per year
Experis Clinical Research Associate
7 salaries
$38.63 per hour
Sunrise Systems Clinical Research Associate
7 salaries
$29.69 per hour
Small Niche CRO! Clinical Research Associate
6 salaries
$88,364 per year
University of Toledo Clinical Research Associate
5 salaries
$66,079 per year
Resource Employment Solutions Clinical Research Associate
6 salaries
$24.04 per hour
RELODE Clinical Research Associate
6 salaries
$5,000 per month
Painter & Associates Personnel Clinical Research Associate
6 salaries
$104,485 per year
Provident Research Inc Clinical Research Associate
6 salaries
$113,374 per year
RBW Consulting Solutions Ltd Clinical Research Associate
5 salaries
$109,280 per year
ParkPower Corporation Clinical Research Associate
5 salaries
$120,496 per year
Acara Solutions Clinical Research Associate
5 salaries
$19.80 per hour
Essentia Health Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$16.73 per hour
New York State Psychiatry Institute Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$45,068 per year
BRIDGEWAY COMMERCE GROUP Clinical Research Associate
5 salaries
$120,496 per year
NuWest Group Clinical Research Associate
5 salaries
$16.43 per hour
Pharmaceutical Clients Clinical Research Associate
5 salaries
$30.00 per hour
Byrnes and Rupkey, Inc. Clinical Research Associate
5 salaries
$120,496 per year
Cap & Sol Clinical Research Associate
5 salaries
$50.00 per hour
RCTS, Inc Clinical Research Associate
5 salaries
$13.15 per hour
All US Jobs Clinical Research Associate
5 salaries
$120,000 per year
The DAVIS Companies Clinical Research Associate
5 salaries
$25.00 per hour
JGB BioPharma Consulting Inc. Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$112,945 per year
Advanced Technology Solutions Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$43.55 per hour
Worldwide Clinical Trials Holdings, Inc. Clinical Research Associate
5 salaries
$109,545 per year
Private Practice Leadership, LLC Clinical Research Associate
5 salaries
$15.71 per hour
Team1Medical Clinical Research Associate
5 salaries
$33,786 per year
ZEISS Group Clinical Research Associate
5 salaries
$48.00 per hour
Worldwide Placement Limited Clinical Research Associate
5 salaries
$90,000 per year
Pharmaceutical Research Associates, Inc. Clinical Research Associate
5 salaries
$76,530 per year
Healthcare Recruiters International Clinical Research Associate
5 salaries
$62,430 per year
Bridgeway Professionals Clinical Research Associate
5 salaries
$120,496 per year
i-Pharm Consulting Clinical Research Associate
5 salaries
Clinical Research Associate Job available
$94,767 per year
Collaborative Clinical Research Associate
5 salaries
$39.65 per hour
The Veritas Healthcare Solutions LLC Clinical Research Associate
5 salaries
$78,850 per year
Louis Stokes Cleveland VA Medical Center Clinical Research Associate
5 salaries
$43,399 per year
Piper Enterprise Solutions Clinical Research Associate
5 salaries
$111,929 per year
Groupware Solutions Clinical Research Associate
5 salaries
$30.00 per hour
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
How to Prepare for a Clinical Research Interview
Preparing for almost any interview may be a stressful experience. When interviewing for clinical research positions, interviews pose a special challenge which may require extra preparation. Below are the following five suggestions to be certain you are correctly prepared for a medical study project interview.
Do your research
First, take a deep dive into a company’s current information, discoveries or updates. LinkedIn is a superb source to observe factors about the business and its workers. Through LinkedIn it is possible to study how long employees work there, their credentials, and their own histories.
In addition, examine the business’ site, take notes and search for any current media releases. Based on your potential situation, your interviewer may would like you to share your own understanding of search practices and suitable protocol. Assessing these practices and describing them will help you through the meeting. For instance, if you're interviewing for a pharmaceutical medical study endeavor, you should take a look at the pharmaceutical sector and any appropriate research that's been published lately.
Get your resume into tiptop shape
The top two aspects clinical research partners look for in a candidate are your qualifications and resume. Your resume functions as your profile, and is also an extension of you. If your resume isn't up-to-date, then you might lose out on chances for possible interviews.
Be certain that you write your credentials into your resume and concentrate on the particular job which you are interviewing for. Examine the job description along with their needs for tips about what info you need to elaborate on, but don't over embellish you achievements.
Print multiple copies of your resume and then maintain them at a professional folder or notebook. Summarize your expertise and goals into a quick elevator pitch, and you’re ready for the next step.
Questions and ANSWERS
Before you go into an interview, it is critical that you prepare for questions the employer might ask. As a exercise, we recommend printing out the exact work description of the job positing and taking a look at every requirement. With every demand, write a vital illustration of how you've had a direct or related experience. Have precise and succinct examples of real life adventures that will assist you to swiftly collect your ideas throughout the interview. This exercise can help you to get accustomed to the position and realize areas where you're a solid candidate.
For a clinical study project interview, your previous clinical study experience is remarkably significant. Be certain you are extremely confident with all the comprehensive information of your previous clinical and research endeavors. Your interviewer may want to ask you certain questions, and you ought to be well equipped to answer. Most importantly, your interviewer will probably ask you about issues that you faced and how you worked together with the remaining part of the study team. Be conscious of occasions when you overcame hardship or faced an obstacle.
In addition answering to their questions, you need to come up with some of your own. This will help demonstrate your interest in the company and position. These questions ought to be unique and should reveal you have completed a thoughtful analysis on company. Make sure these questions are genuine and show a real interest in the employers. However, steer clear of questions regarding benefits, time bonuses and off. These questions can make you to look as though you believe that a provider owes you something, even though this is not the case.
Review your previous research
Walking into a meeting unprepared is essentially requesting collapse and also a missed job prospect. Interview preparation may be tiring and stressful, but the rewards are immense. Simply take the aforementioned five measures seriously, and you're on your path to a thriving clinical study project interview.
Prior to your clinical study job interview, put aside a couple of hours to gather your ideas. To begin, make sure you know who you are meeting . Locate them via LinkedIn and learn a little more about them. After that you can examine their own career path and create rapport more easily.
As mentioned above, along with your resume, be certain you are completely honest throughout this meeting. The job market can be challenging and you might be tempted to embellish a little, but we strongly urge you against that. During your interview, you could be caught in a lie, therefore entirely destroying your odds or, worse, you might get hired and then you're not able to complete the tasks you promised to be experienced in.
Lastly, get certified to make a strong candidate through CCRP Course.
At CCRPS.org, we offer seven courses and certification trainings to give you an advantage. 82% of our students are hired within the first month of taking the course. We are accredited by the Accreditation Council For Clinical Research & Education (ACCRE) and tailor our course to you. For example we offer special courses for nurses and an accelerated certification + internship opportunity for anyone with minimal or no clinical experience.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
How to Improve My AMCAS Work & Activities
Below are five simple ways you can improve your AMCAS Work & Activities
CCRPCOURSE.COM
Educate where you can
CCRPCOURSE.COM allows premeds in their gap year to be certified as Clinical Monitors to pursue a job where they have have expense-covered travel and oversee clinical trials run in medical schools around their region.
Expand your system
As a rigorous and open minded health care practitioner, you may be wondering exactly what you have to do in order to scale the corporate ladder quicker in the medical market. While the principle of'practice makes perfect' uses in the medical sector just like every other profession, progressing your health care profession will need more than your art in the business.
Remember that as you expand and grow, the exact same occurs for your health care career. The five projects highlighted below are the very first couple of actions that will assist you to get a location you would like from the area of medicine. Practice them, along with your healthcare profession will expand also.
Are your interests Dealing with your career route? Here is the very first question you need to have the ability to answer frankly in the event that you would like to improve your career in the health care market. Furthermore, if you're still not in the health care field, you need to ask yourself whether you may achieve the skills and instruction together with the experiences required to realize your objective. You might even elect for clinical research partner training for a means to improve your own comprehension.
Make the most of the onsite courses in addition to any instruction opportunities being provided. Besides that, additionally, there are hospital control and Clinical Research online classes being supplied at some prestigious schools, including McMaster University and James Lind Institute. As previously stated, choosing a clinical research partner instruction is a wonderful method to raise your probability of progressing since the longer you improve, the further you're generating opportunities for your own.
Stay ready, driven and motivated
You always should stay concentrated on the job at hand and being ready for everything and anything that comes your way. It is a very good method of demonstrating that you just take your career seriously. Regardless of what the job you're accountable for, whether large or little, always be ready for it. Additionally, don't forget to get centered on your potential career goals too, even though it feels like the progress you would like is farther away than you originally envisioned. Maintain those friends that are interested in exactly precisely the exact identical field near. You are able to think about these as motivators who drive you to keep on chasing your dream.
Picking a mentor has a few benefits, that will, in turn, assist you in improving your health care career. If you locate a suitable individual that's eager to take you under their wing, then begin with taking a good look at the job ethics and degree of knowledge. This will definitely point you in the ideal way, as you'll be asking yourself how you are able to be like these. Second, your mentor might assist you to network with other seasoned professionals who he/she understands.
Your present or former teacher from medical college will even share his wisdom and adventures with you. This may be a fantastic place to look for out information on whatever that you would like to learn regarding your health career. Utilizing the adventures of your mentor or boss since your aims may be the simplest route to follow if you would like to advance professionally.
Re-evaluate your livelihood
Another great approach to progress your career is by simply linking with likeminded people who work as caregivers. These professionals are available literally anywhere -- the establishment you've have your health care degree from, in your current (and past ) job, in seminars and conventions, in LinkedIn classes, etc.. ) If you would like to branch out farther than the men and women in your institution, you can attempt using online social networks to associate with more people just like you. Conventional social networks like working on your area and associations also work miracles.
Applying to medical school can be difficult, but it can also be one of the most important decisions of your life. The AMCAS is the American medical school application system that all students use. A rich, well put together application can help you get into your dream school. Below are four simple ways you can improve your AMCAS Work & Activities
Expand your system
As an aspiring health care practitioner, you may be wondering exactly what you have to do in order to scale the corporate ladder. While improving your skills and practice helps your career in the medical sector as well as every other profession, progressing your health care profession will need more than perfect bedside manners.
Remember that as you expand and grow, the exact same occurs for your health care career. The five questions highlighted below are the guide to the first actions that will assist you to get a location you would like from the area of medicine. Practice them, along with your healthcare profession will expand also.
Are your interests Dealing with your career route? Here is the very first question you need to have the ability to answer frankly in the event that you would like to improve your career in the health care market. Furthermore, if you're still not in the health care field, you need to ask yourself whether you may achieve the skills and instruction together with the experiences required to realize your objective. You might even elect for clinical research partner training for a means to improve your own comprehension.
Make the most of the onsite courses in addition to any instruction opportunities being provided. Besides that, additionally, there are hospital control and Clinical Research online classes being supplied at some prestigious schools, including McMaster University and James Lind Institute. As previously stated, choosing a clinical research partner instruction is a wonderful method to raise your probability of progressing since the longer you improve, the further you're generating opportunities for your own.
Stay ready, driven and motivated
You always should stay concentrated on the job at hand and being ready for everything and anything that comes your way. It is a very good method of demonstrating that you just take your career seriously. Regardless of what the job you're accountable for, whether large or little, always be ready for it. Additionally, don't forget to get centered on your potential career goals too, even though it feels like the progress you would like is farther away than you originally envisioned. Maintain those friends that are interested in exactly precisely the exact identical field near. You are able to think about these as motivators who drive you to keep on chasing your dream.
Picking a mentor has a few benefits, that will, in turn, assist you in improving your health care career. If you locate a suitable individual that's eager to take you under their wing, then begin with taking a good look at the job ethics and degree of knowledge. This will definitely point you in the ideal way, as you'll be asking yourself how you are able to be like these. Second, your mentor might assist you to network with other seasoned professionals who he/she understands.
Your present or former teacher from medical college will even share his wisdom and adventures with you. This may be a fantastic place to look for out information on whatever that you would like to learn regarding your health career. Utilizing the adventures of your mentor or boss since your aims may be the simplest route to follow if you would like to advance professionally.
Re-evaluate your livelihood
Another great approach to progress your career is by simply linking with likeminded people who work as caregivers. These professionals are available literally anywhere -- the establishment you've have your health care degree from, in your current (and past ) job, in seminars and conventions, in LinkedIn classes, etc.. ) If you would like to branch out farther than the men and women in your institution, you can attempt using online social networks to associate with more people just like you. Conventional social networks like working on your area and associations also work miracles.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research Career Salaries (CRA, CTA, CRC, ect)
CCRP Course Provides Certification to work in the following roles
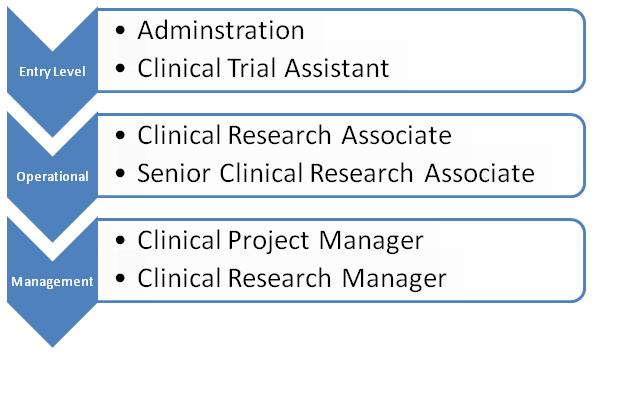
Senior Clinical Research Associate (CRA) 200 profiles
$101,978 $74K-$121K
Clinical Research Associate (CRA) 198 profiles
$67,532 $44K-$106K
Clinical Trial Manager 56 profiles
$102,283 $69K-$139K
Clinical Project Manager40 profiles
$111,006 $73K-$136K
Clinical Operations Manager 7 profiles
$116,859 $108K-$125K
Clinical Research Manager 6 profiles
$108,250 $98K-$119K
Associate Clinical Research Director 5 profiles
$136,950 $116K-$148K
The tuition fee is $1,795 ($18 per module) is offered over a 4-month payment plan in this link. The online training can be completed within 1-4 months time and can start within 24 hours of tuition payment.
Are you looking to work in the clinical research field? CCRP Courses provides certification to work in the following roles:
Senior Clinical Research Associate (CRA)
$101,978 on average, from $74K-$121K
Clinical Research Associate (CRA)
$67,532 on average, from $44K-$106K
Clinical Research Coordinator (CRC)
$50,451 on average, from $38K-$68K
Clinical Trial Assistant (CTA)
$45,412 on average, from $31K-$66K
$102,283 on average, from $69K-$139K
$111,006 on average, from $73K-$136K
$116,859 on average, from $108K-$125K
$108,250 on average, from $98K-$119K
Associate Clinical Research Director
$136,950 on average, from $116K-$148K
The tuition fee is $1,795 ($18 per module) is offered over a 4-month payment plan in this link. The online training can be completed within 1-4 months time and can start within 24 hours of tuition payment. Our courses are ACCRE accredited and curated by field professionals that know what they are doing. Click below to learn more about clinical research positions.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
CCRPS Clinical Research Certificate Program Overview
This program enables you to master practical aspects of clinical trial conduct and management. For more info read our blog.
The First Step to Clinical Research Certificate Program
_________________________________________________________________________________
CCRP CRRA Certified Clinical Researcher Associate
This program requires only a medical sciences education background such as FMGs, BSNs, and Bachelors graduates. Clinical Research Associate Training Course is a 110 module course led by Senior CRA and Physician of 25 Years, Dr.Kamal. This course provides the training, experience, interviewing, letter of references, and connections you need to get a job supervising clinical research efforts. We offer clinical research associate training and placement for nurses, science bachelor graduates, and international medical graduates.
_________________________________________________________________________________
SCDM CCDM Certified Clinical Data Manager
Eligibility Requirements
APPLICANTS FOR THE CERTIFIED CLINICAL DATA MANAGER (CCDM™) PROGRAM MUST MEET ONE OF THE FOLLOWING CRITERIA
Bachelor’s degree or higher and minimum two years full-time CDM experience
Associate’s degree and minimum three years full-time CDM experience
Four or more years full-time CDM experience
Part-time work experience equal to or surpassing full-time equivalent in criteria above
ACRP CCRA Certified Clinical Research Associate
The CCRA® eligibility criteria define the minimum experience required before a CRA can apply for the program. It is important to compare the CRA eligibility criteria with your career and educational experiences to self-determine your eligibility before submitting an application and payment. All applications must undergo a formal review process to determine an applicant’s eligibility to sit for the exam.
CRA Certification Eligibility Requirements
In order to be deemed eligible to take the CCRA® exam, applicants for the CCRA® credential must be able to provide evidence through a job description, detailed CV, or other documentation that they:
Work independently of the investigative staff conducting the research at the site or institution. This means they do not report to the PI or site manager and that they do not have the ability to change or manipulate data, and;
Work on behalf of the sponsor. This means that they are contracted by the “sponsor” to perform an independent monitoring function. The “sponsor” can be a pharmaceutical or device company, a granting agency, a university department, a physician, etc., and;
Perform all of the CCRA® essential duties as detailed below for a required minimum number of hours. Hours performing the CRA essential duties can include hoursdocumented up to the date of the exam and/or through previous employment. The required number of hours is dependent upon one’s educational background.
The CRA Certification Handbook has complete information on eligibility requirements.
ACRP CCRC Certified Clinical Research Coordinator
CRC Certification Eligibility Requirements
In order to be deemed Eligible to take the CCRC® exam, applicants for the CCRC® credential must be able to provide evidence through a job description, detailed CV or other documentation that they:
Perform all of the CRC essential duties as detailed below for a required minimum number of hours.
Hours performing the CRC essential duties can include hours documented up to the date of the exam and/or through previous employment. The required number of hours is dependent upon one’s educational background.
SoCRA CCRP Certified Clinical Research Professional
The applicant must be working with Good Clinical Practice (GCP) guidelines under IRB/IEC/REB-approved (or specifically exempted) protocols.
The applicant must meet one of the following Eligibility Criteria noted below. Please note that most candidates will be eligible under Category 1.
For purposes of eligibility, please view SOCRA's definition of a Clinical Research Professional.
If you have a question about Candidate Eligibility please email SOCRA at certification@socra.org
Category 1
Applicant must have (and be able to document) ALL of the following qualifications:
Have two years of experience as a full-time Clinical Research Professional (or have 3,500 hours part-time) during the last five years
Please note: If you have completed two (2) years of full-time employment as a clinical research professional in the past five years, you will NOT need to provide supporting documentation for your educational experience.
Category 2
Applicant must have (and be able to document) ALL of the following qualifications:
Hold a degree in "Clinical Research" from an Associate, Undergraduate, or Graduate Degree Program AND
Have completed a minimum of one year of full-time experience (or 1,750 hours part-time) during the past two years as a Clinical Research Professional
Category 3
Applicant must have (and be able to document) ALL of the following qualifications:
Hold an Undergraduate or Graduate Certificate in “Clinical Research” with a curriculum of no less than 12 semester (credit) hours or totaling a minimum of 144 credit hours from an academic institution of higher learning (community college, college or university) AND
Hold an Associate’s or Bachelor’s Degree in a science, health science, pharmacy or related field AND
Have completed a minimum of one year of full-time experience (or 1750 hours part-time) during the past two years as a Clinical Research Professional.
ACRP CCTI Certified ClinicPrincipal Investigator
The CPI® eligibility criteria define the minimum experience required before a PI can apply for the program. It is important to compare the CPI® eligibility criteria with your experience to self-determine your eligibility before submitting an application and payment. All applications must undergo a formal review process to determine an applicant’s eligibility to sit for the exam.
PI Certification Eligibility Requirements
In order to be deemed eligible to take the CPI® exam, applicants for the CPI® credential must be able to provide evidence through a job description, detailed CV or other documentation that they:
Have a doctorate level degree and;
Have proof of employment as a PI during at least two (2) of the most recent five (5) years and;
Perform all of the essential duties as detailed below.
The PI Certification Handbook has complete employment definitions.
Update: The CPI Exam is the first exam being offered to all investigators with a doctoral-level degree. A license to practice medicine is no longer required.
NAIM CIM Certified IRB Manager
To qualify for the Certified IRB Manager Examination, you must meet the following
requirements:
• Applicants must have a Bachelor’s Degree and 2 years of relevant IRB
Management experience within the last 5 years.
OR
• An Associates Degree from a two year college or technical school and 2 years
of relevant IRB Management experience within the last 5 years.
CCIP CIP Certified IRB Professional
Eligibility Requirements
This certification program is for individuals whose primary job responsibilities include substantial participation in overseeing, administering or performing the daily activities of an IRB as part of a HRPP. Individuals involved in IRB activities who meet the following eligibility requirements are eligible to take the examination:
A Bachelor's degree plus two years of relevant HRPP experience, completed on or before the first day of your chosen testing period (see front cover)
or
Three years of relevant HRPP experience, completed on or before the first day of your chosen testing period
or
Current certification as a CIP
RACC CRA Certified Research Administrator
Bachelor’s Degree and three (3) years of professional experience in research or sponsored programs administration either in a sponsoring or recipient organization or the equivalent in a self-funded organization;
OR
An Associate’s Degree and five (5) years of professional experience in research or sponsored programs administration either in a sponsoring or recipient organization or the equivalent in a self-funded organization;
OR
No degree and six (6) years of professional experience in research or sponsored programs administration either in a sponsoring or recipient organization or the equivalent in a self-funded organization. *
MAGI CRCP Certified Research Contract Professional
People often ask how to prepare for the exam. Two years of experience with a variety of negotiation partners may be sufficient to pass the exam. A careful reading of MAGI's Model CTA, Clinical Trial Agreement Handbook (Original), and Model CDA is helpful. There are numerous relevant articles in the Journal of Clinical Research Best Practices. Attending MAGI's Clinical Research Conference is good preparation, provided you attend sessions and workshops that address relevant topics where you need more knowledge. Sample questions, study guides, or other study materials are not available. However, imagine an exam for professional chefs. One question might be, "Describe the process of making an omelette." Another might be, "List five spices used in Chinese cuisine." Another might be, "What are the pros and cons of gas vs. electric stoves?"
RAPS RAC Regulatory Affairs Certification
The RAC is the only credential for regulatory professionals in the healthcare product sector. The RAC demonstrates to employers, clients and colleagues essential knowledge, critical thinking abilities and a commitment to continuing professional development. It is designed for working regulatory professionals, with at least three to five years of regulatory experience. There are six different RAC exams. The US, EU and Canada exams test regional regulations and involvement with regulatory bodies. The global exam focuses on international standards and guidelines. The devices and drugs exams have a global focus yet aligns to a specific sector of regulation knowledge. All six exams test for regulatory knowledge, critical thinking and analysis throughout the lifecycle of a product.
SQA RQAP Registered Quality Assurance Professional
The Society of Quality Assurance is proud to offer the professional credential Registered Quality Assurance Professional (RQAP) for professionals working in Good Laboratory Practices as well as for professionals working in Good Clinical Practices. Registration is a recognized standard of experience and knowledge throughout the QA industry, in the US and around the world.
What the credential demonstrates
Proof of your knowledge of the regulations/guidelines and how they are applied
Commitment to a high quality standard in the QA industry
Personal professional growth and achievement
How it Works
Professionals must pass an examination in either Good Laboratory Practices or Good Clinical Practices to receive the RQAP credential.
Registered professionals must re-register every three years by submitting documentation of ongoing professional activities. These activities include, but are not limited to:
Attending the SQA Annual Meeting and/or Quality College
Attending events of SQA Regional Chapters
Attending events of relevant professional associations/societies, e.g. JSQA, RQA, RAPS, DIA, ACS, ACRP, NAICC, AALAS, etc. (See our MOU Organizations and Liaison Organizations.)
Completing courses in the SQA Online Learning Center
Attending SQA webinars or webinars offered by relevant professional organizations
Studying the SQA Annual Meeting Online Library
Participating in the SQA Mentoring Program
Writing test items for an RQAP exam
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
From The Course Creator: CRA Clinical Research Certification & Training
CRAs can expect to make between $75-90k/year within their first year. CRAs who are physicians from foreign countries are considered MDs in this position. CRAs who are nurses which transitioned are treated remarkably well among the hospitals they visit or monitor. As a supervisor of clinical trials, this also paves ways to enter higher-level administrative and leadership roles in medical centers, institutions, and pharmaceutical companies.
Clinical research associates are currently the highest paid upper-level medical professionals in the pharmaceutical and medical industry, second only to physicians. Many remote and traveling (expenses-covered) jobs are available for CRA positions for those who have a background or certification training.
The obvious difficulty in searching for these positions is getting selected and interviewed without experience. CCRP Course works directly with:
International Medical Graduates (MBBS, FMG, IMGs)
MDs without residence
Bachelor’s of science or healthcare-related fields
Bachelor’s of nursing and nurses
Master’s of science graduates
CCRPS’ courses strives for you to achieve the knowledge, certification, and resume experience needed to be selected and hired by pharmaceutical companies.
CRAs can expect to make between $75-90k/year within their first year. CRAs who are physicians from foreign countries are considered MDs in this position. CRAs who are transitioned nurses are treated remarkably well among the hospitals they visit or monitor.
As a supervisor of clinical trials, this position also paves ways to enter higher-level administrative and leadership roles in medical centers, institutions, and pharmaceutical companies.
Courses for Quality CRA Training Modules per Course
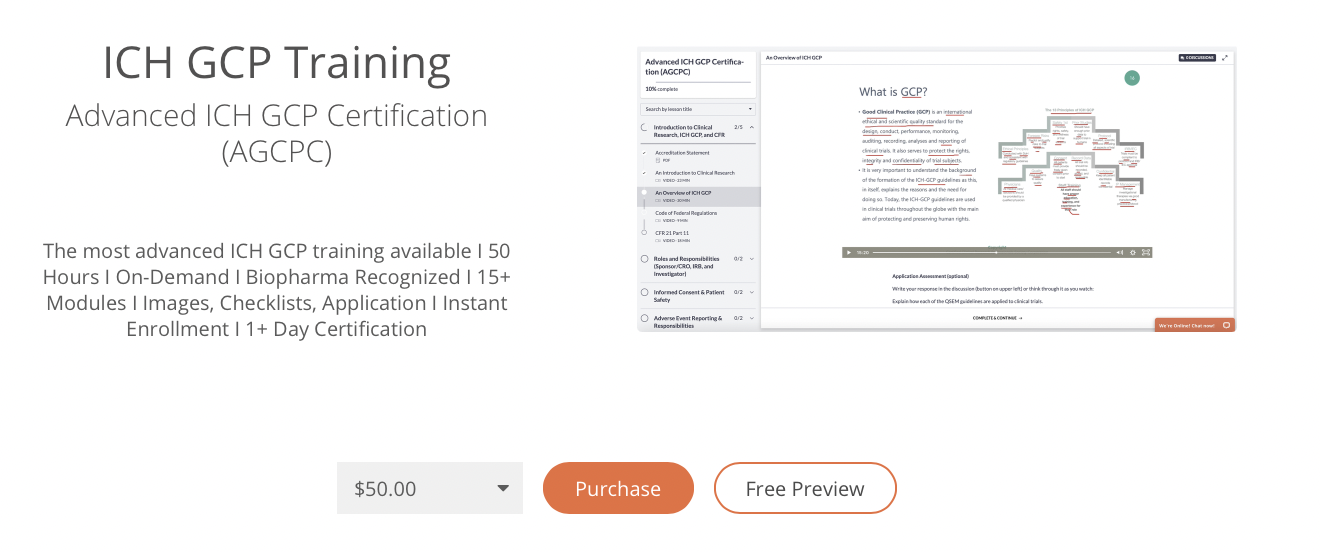
1. ICH GCP Training 15
2. Quality Monitoring 45
3. Regulatory Training 15
4. Audit and Inspections 6
5. Writing Professional Monitoring Reports 16
-Developing Follow Up Reports
6. Subject Recruitment Retention and Compliance 5
7. Misconduct and Fraud 2
8. Competency Testing of CRA’s 6
………… Total 110 Modules
Training certification will be provided at the end of the course. This can be listed on your resume as an educational experience as well as a volunteer experience for the amount of time it takes you to complete the course.
The tuition fee is $1,795 ($18 per module) is offered over a 4-month payment plan in this link.
The online training can be completed within 1-4 months time and can start within 24 hours of tuition payment.
After module completion we begin your recruiter-interaction training by providing the following:
1. Interview Prep (3 interview rounds training)
2. Resume experience of training
3. Recruiter based references
4. Multiple employee references
5. LOR’s
Having been a Senior CRA for 15 years, my job is to ensure that entering and succeeding this field is easier for our students. If you are interested in this field, below are some CRA articles you may find useful.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
About CCRPS
CCRPS provides clinical research certification through ACCRE, ACPE, ANCC, AMA accredited courses for clinical research associate certification, clinical research coordinator certification, and clinical research assistant certification online. Request a partial scholarship or payment plan options and get started on your course right away.
CCRPS Clinical Research Certification
Certified Clinical Research Professionals Society (CCRPS) educates the drivers of clinical trials research worldwide through our certification program. We are a Virginia-based 501(c)(3) not-for-profit professional organization serving all people involved in clinical trials from volunteers to professionals. Our mission is to support clinical trials by providing accessible education to entry and mid level professionals.
As part of our duty to provide accessible education, we offer free ICH GCP certification (good clinical practice gcp) recognised by Transcelerate Biopharma for all students, businesses, and professionals.
We provide clinical research associate, clinical research coordinator, clinical research assistant, ICH GCH, pharmacovigilance drug safety, and international clinical research certifications accredited and peer reviewed by the ACCRE. Healthcare professionals can also receive ACPE, AMA/ACCME, ANCC, and ICPE continuing education credits through our clinical research online training.
Learn more about our cra certification through our clinical research associate certificate.
Get answers to your questions regarding the online course immediately by chatting with our team online. You can demo all of our courses for free by clicking below.
Our Clinical Research Courses
CCRPS is uniquely positioned to provide accredited certification courses for entry level professionals due to our in-depth content. All courses can be accessed immediately after enrolling. Students have 2 attempts to pass the certification exam with a minimum of 70% in order to receive their certificate. Our courses are are entirely online, self-paced, and can be completed in between 1 week - 12 weeks, depending on the content matter and your schedule. Students can apply for partial scholarships or enroll for a payment plan for CRA and CRC certification courses. To get more information regarding our partial scholarship, chat with our enrollment agents online. Scroll down to learn more about our accreditation, certification, and CME credit provisions.
Clinical Research Training and Job Placement
CCRPS, like other educational institutes, is only associated with educating and certifying clinical research professionals, so we do not provide job placement. However, we want to make sure you apply with your best foot forward. Below are links we readily refer to graduates who are looking for job support. Having a great CV and cover letter are essential to applying for jobs. Recruiters are paid by the company which hires you and thus are free for searching employees. Be realistic but also be driven. Make sure you reach out if you received no response, as the company may never have seen your application.
Clinical Research Job Advising: Kunal at ClinicalTrialPodcast
Free Resume Review: TopCV TopCV provides a free review and feedback for your current resume.
Resume Distribution: ResumeRabbit Resume rabbit distributes your resume to 60 job posting sites.
Clinical Research Recruiters: I-Recruit I-Recruit distributes your resume to clinical research recruiters.
Clinical Research Job Bulletin: Indeed Indeed usually provides the most uptodate job bulletin for clinical research jobs
CCRPS FAQS
CCRPS Reviews and Testimonials
CCRPS has trained over 1,700 professionals to date. Our students continue to provide us great feedback through email, online, and other methods. Below are some audio and video testimonials students have shared with us. Students benefit from hearing what past students have to say as well as trying the course demo online.
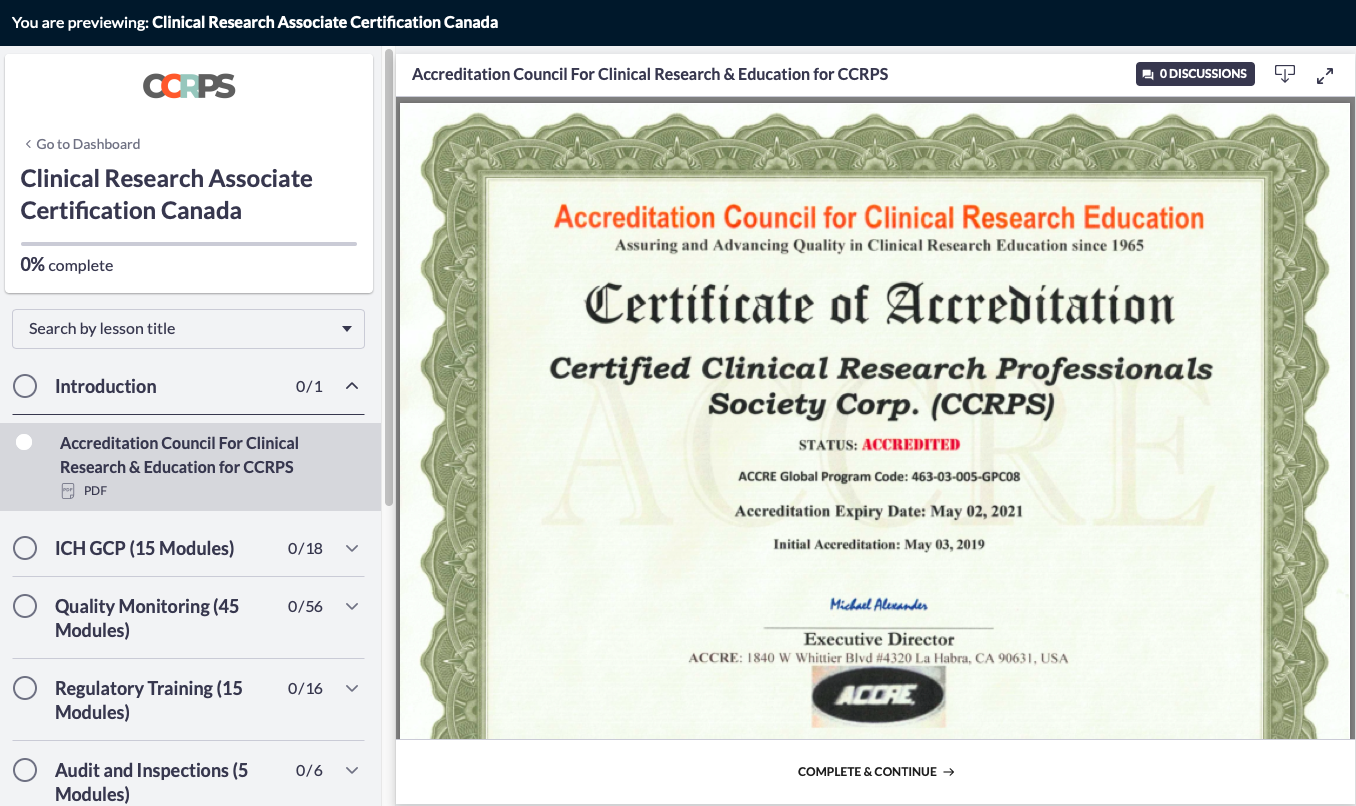
Accreditation for CCRPS Courses
ACCRE - CLINICAL RESEARCH PROFESSIONALS
CCRPS CRA Certification and CRC Certification is accredited by The Accreditation Council for Clinical Research Education (ACCRE) in June 2019 Code: 463-03-005-GPC08
ACCRE accredits professional programs in Clinical Research leading to the Certificate of Clinical Research. The ACCRE is recognized for the accreditation and pre-accreditation of professional programs in Clinical Research by the biotechnology and pharmaceutical industries. ACCRE accreditation serves to establish standard eligibility for participation in a variety of biopharmaceutical industry funded programs. For students and prospective students, accreditation provides an assurance that a program has been found to provide satisfactory educational preparation for practice in the field
JOINT ACCREDITATION - PHYSICIANS, NURSES, PHARMACISTS, HEALTHCARE PROVIDERS
Advanced Clinical Research Professionals Foundations (ACRP-F)
Release date: 07/14/2020 Expiration date: 07/14/2021
Estimated time to complete activity: 17.5 hours
Jointly provided by Postgraduate Institute for Medicine (PIMED) and Certified Clinical Research Professionals Society (CCRPS)
This activity is intended for physicians, nurses, pharmacists, and professionals engaged in care of patients in clinical trials.
Educational Objectives
After completing this activity, the participant should be better able to:
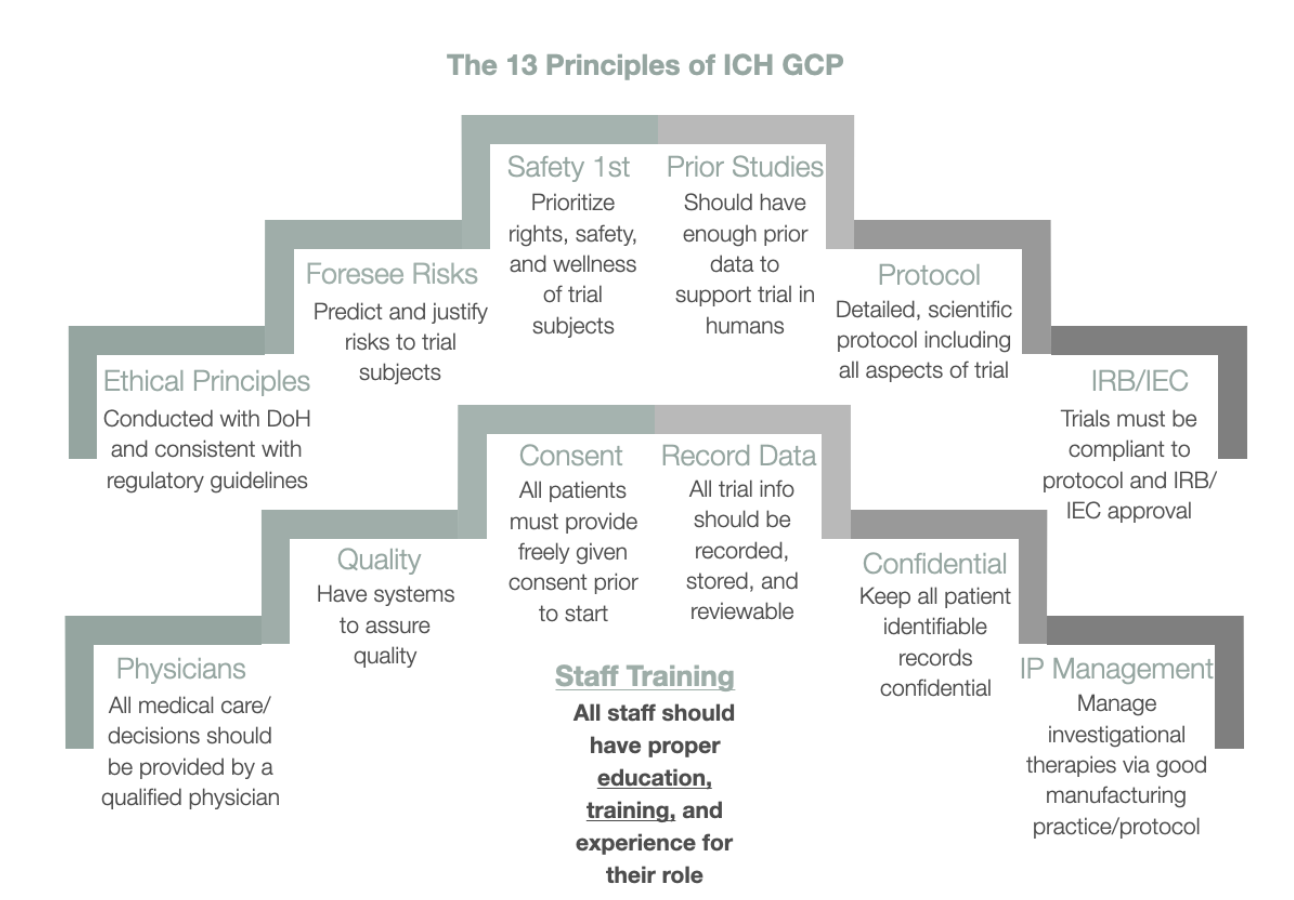
Describe the ICH GCP protocol including FDA 21 CFR, E2A, 5,5 E6, and Ethics
Explain the Quality Monitoring process including designing trials, site visits, monitoring visits, close out visits, protocols, and reporting violations.
Outline the process and professional roles involved in audit and inspections.
Appropriately detect and report misconduct and fraud.
Apply concepts of clinical research roles in a multiple choice examination.
Faculty
Dr.Kamaljit Tiwana, Diedre Clarke, Sahar Khan
Program Agenda
This program is self-paced and can be completed at the time desired by the student.
Joint Accreditation Statement
In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Certified Clinical Research Professionals Society (CCRPS). Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
ACCME / AMA - PHYSICIAN CME
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 17.5 AMA PRA Category 1 Credit(s)™ (CME). Physicians should claim only the credit commensurate with the extent of their participation in the activity.
ACPE - CONTINUING PHARMACY EDUCATION
Postgraduate Institute for Medicine designates this continuing education activity for 17.5 contact hour(s) (17.5 CEUs) of the Accreditation Council for Pharmacy Education.
Universal Activity Number - JA4008162-9999-20-2166-H04-P
Type of Activity: Knowledge
ANCC - CONTINUING NURSING EDUCATION
The maximum number of hours awarded for this Continuing Nursing Education (CNE) activity is 17.5 contact hours.
ICPE - HEALTHCARE PROVIDERS INTERPROFESSIONAL CONTINUING EDUCATION
This activity was planned by and for the healthcare team, and learners will receive 17.5 Interprofessional Continuing Education (IPCE) credit for learning and change.
TRANSCELERATE BIOPHARMA - ICH GCP
Recognizes CCRPS Certification as Evidence of GCP Training. CRA, CRC, and ACRP-F certification available by CCRPS meet the criteria for GCP training. We are recognised by the 20 major TransCelerate associated BioPharma companies through mutual-recognition to prevent overlap in GCP training.
Clinical Research Training For Nurses: A Guide to Becoming a Clinical Research Nurse
CLINICAL RESEARCH TRAINING FOR NURSES
Guide to Becoming a Clinical Research Nurse
What is Clinical Nursing Research?
Nurses are known for providing direct care for patients. However, nurses may take up roles that are completely new to them within the world of clinical research. These roles include clinical research coordinator, educator and manager. They can also take up less traditional role like regulatory specialist, study monitor and IRB (institutional board review) admin.
Regulatory specialist: their activities relate mainly with preparing regulatory documents and communicating with regulatory bodies. Nurses can work as a regulatory affairs specialist, a regulatory operation coordinator, or a regulatory coordinator. They can work within government agencies, pharmaceutical companies, academic medical centers.
Study monitor: they monitor clinical research practices and make sure that it complies with necessary research protocols and regulations. They tend work at government agencies, biotechnology companies, pharmaceutical companies, contract research organizations, device manufacturers etc.
Institutional Review Board (IRB) administrator: they are the professionals in charge of overseeing, administrating, implementing and managing IRB activities, like policies and procedures that relates to protecting human welfare. They can work at all IRBs: local, commercial or central IRB.
Nurses that have developed interest in the field of clinical research can join professional organizations. This provides them with the opportunity to network and continue their education through mediums like conferences, webinars, discussion groups, publications and online resources. These avenues serve as part of their clinical research training.
Certification is often a parameter used to measure professional expertise. This is based on criterion that reflects skill, knowledge, educational preparation, ability, and competence that are developed from experience in that area of specialization. Nurses that developed an interest in clinical research and have taken a clinical research training program have an opportunity to be certified through the:
Society for Clinical Research Professionals, Inc. (Certified Clinical Research Professionals)
Association for Clinical Research Professionals (Certified Clinical Research Associate or Certified Clinical Research Coordinator)
This field of clinical research gives nurses a chance, an opportunity to advance themselves professionally in a field that might not have been explored by them before. The benefits of having a registered nurse cover letter are insurmountable. This also provides a career path that can show family members the benefits of working in the medical field.
Nurses that have gone through the clinical research for nurses, otherwise called research nurse can carry out research on the various aspects of the human health, such as illness, pharmaceutical and health care methods and treatment plans. The main aim of this research is to improve the quality of health care service delivery. Helping patients and their family in a healthcare facility also brings a level of joy that is hard to find in many other career paths.
Roles of Research Nurses
They are responsible for designing and implementing research studies.
They observe procedures for treatment, collect and analyze data.
They report their research results to appropriate quarters.
They write articles and report their research findings in nursing or medical professional publications and journals.
They help in recruiting participants for studies and are involved in providing direct care for the participants.
Clinical research nurse salary can make use of their communication skills as well as their critical thinking skills gotten from their knowledge and experience in healthcare to further their career in this exciting way.
Know that future CRNs can speak to our 24/7 chat and phone advisors to request information on partial scholarships and payment plans for nurses.
2. Clinical Research Nurse Salary
The average pay for a Clinical Research Nurse is $31.28 per hour.
MD Anderson Cancer Center Clinical Research Nurse salaries - $71,503/yr
Northwestern University Clinical Research Nurse salaries - $75,005/yr
NIH Clinical Research Nurse salaries - $77,331/yr
CLINICAL RESEARCH NURSE JOB Description
A clinical research nurse conducts scientific research on different aspects of human health like illnesses, pharmaceuticals, treatment plans and healthcare methods. Their major goal is to improve the quality of healthcare services that are administered to the patients.
3. How do I get Clinical Research Nurse Experience?
Experience don’t just jump on you, you have to get it by practice. CCRPS affords you an opportunity to acquire knowledge in clinical research, and not just knowledge but experience as well. Registering for the appropriate course will boost your knowledge base and as well you get experience of clinical research first hand.
As a clinical research nurse, you will be at the forefront of new medical discoveries, and help develop breakthrough cures and medical treatments. The work that you do during your career can help some patients live longer or better quality of life. You may be responsible for studying diseases and disorders, as well as developing new treatment plans. You will also help test new treatments and medications that could possibly change the way a disease or disorder is perceived.
The field of clinical research can be very rewarding and fulfilling. A good research nurse is dedicated to their work and ready to take on everything that the profession throws their way. If you’re looking to pursue a research nursing career, you should have an excellent understanding of the research process as well as the specialty area that you’re studying.
Excellent communication skills are also a must. You must be able to effectively communicate with scientists, physicians, researchers, patients, and corporate executives.
4. What Does a Clinical Research Nurse Do?
The duties of a research nurse will typically depend on their employer and role. Some research nurses may be responsible for studying diseases, while others may help create and improve new medications and other treatments.
clinical research nursing scope and Standards of Practice
Clinical research nurses can take up clinical research jobs in institutions like research organizations, pharmaceutical companies, universities, research laboratories, government agencies and teaching hospitals.
The work that a research nurse does is quite exhaustive and it includes;
They use their knowledge of the basics of clinical research in designing and implementation of research studies.
Observation of the procedures for patient treatment, collection and analyzing of data.
They report their research findings to the relevant authorities. They may also have to present their results at health conferences and publish them in journals.
They write grant applications in order to secure funds to carry out the research.
They render assistance in the process of recruiting study subjects.
They provide direct treatment for research participants.
Research nurses that study diseases and illnesses will often perform a great deal of research, both by studying previous findings and observing patients. They may be required to examine medical journals, for instance, as well as observe, study, and care for patients suffering from a particular disease.
They make decisions based on the observations made as to which patients are the best candidates for certain clinical trials. During clinical trials, the research nurse will administer medications or perform other treatment procedures, During this process, research nurses must closely monitor each patient’s progress. This includes documenting side effects, drug interactions, and the overall efficiency of the medication.
Aside from caring for patients, documenting and recording information during clinical trials are the most important responsibility that a research nurse has. The information and data gathered during the research must be compiled into reports and handed over to senior clinical researchers or specialists.
5. How Do I Become a Research Nurse?
Don’t expect to become a research nurse overnight. It's a lot of work and you are expected to undergo years of training and experience.
The clinical research nurse job is a competitive one and certificates are not just handed out to anybody. The conditions to be eligible to take the certificate exam is that you must be an experienced registered nurse and your experience must include having thousands of hours of experience in the area of clinical research.
How to Become a Registered Nurse (RN) in 2020 that contains everything a person pursuing a nursing job should know - responsibilities, education, salaries and more.
The first step toward becoming a research nurse is to obtain a proper education. You can start with a bachelor’s degree in nursing, although many employers prefer that their research nurses have master’s degrees or even doctoral degrees in their chosen specialty. During your schooling, classes in research and statistics are a must and are courses in your chosen area of expertise.
According to clinical research job websites, many research nurses have a MSN degree and some have a PhD in nursing. Many of them attain these degrees of education in order to give them an edge on getting clinical research positions. While studying, courses in statistics and research are mandatory.
There are two main certifications that clinical research nurses can get from the Association of Clinical Research Professionals (ACRP). You can get certification to become a certified clinical research associate or you can choose to become a certified clinical research coordinator.
Take courses from CCRPS and learn more on how to become a clinical research nurse.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
6. Clinical Research Nurse Requirements and Certifications & Nursing Cover Letter
A bachelor's degree in nursing does meet licensure requirements for graduates to become registered nurses (RNs), which qualifies individuals for the specialized certification. Bridge programs, such as an RN-to-Bachelor of Science in Nursing (BSN), require previous nursing education for admission. Nursing students complete traditional classroom courses, laboratory experiences, and a clinical practicum in a medical setting, which includes a hospital, assisted living facility, and long term care center.
For specific education in clinical research, trained RNs enroll in graduate certificate and degree programs. There students are introduced to case studies, ethical research practices, and financial matters affecting the design, implementation, and funding of clinical research trials. In a master's program, studies in research ethics point students towards ethical research practices, including a discussion on human rights, misconduct, and conflicts of interest. Graduate programs will also include quantitative research and a capstone project.
All RN-to-BSN programs will require an RN license to enroll. Master's and graduate certificates will need a bachelor's degree with sufficient prerequisite coursework in the field. In addition, they will need letters of recommendation or reference, a personal statement, and GRE scores.
Becoming a nurse researcher which is a highly specialized career requires an advanced degree and training in informatics and research methodology and tools. The initial step for these individuals, or for any aspiring advanced practice nurse, is to earn a Bachelor of Science in Nursing degree and pass the NCLEX-RN exam. Once a nurse has completed their degree and attained an RN license, the next step is to complete a Master's of Science (MSN) in Nursing program with a focus on research and writing. MSN courses prepare nurses for a career in research and usually include coursework in statistics, research for evidence-based practice, design and coordination of clinical trials, and advanced research methodology.
A TYPICAL JOB POSTING FOR A RESEARCH NURSE POSITION WOULD LIKELY INCLUDE THE FOLLOWING QUALIFICATIONS, AMONG OTHERS SPECIFIC TO THE TYPE OF EMPLOYER AND LOCATION:
MSN degree and valid RN license.
Experience conducting clinical research, including enrolling patients in research studies, Implementing research protocol and presenting findings.
Excellent attention to detail required in collecting and analyzing data.
Strong written and verbal communication skills for interacting with patients and reporting research findings.
For a person to practice nursing legally, acquiring of nursing credentials and certifications is very important. For instance, some nurses who achieve a master's degree (MSN) leave the patient care aspect of nursing, and practice in a more managerial role.
CRA JOB OPPORTUNITIES
If you choose to become a Clinical Research Associate (CRA), you will have a key role in the success of clinical trials. Most CRAs have a nursing background, like yours. You will be the primary contact and support for trial sites, ensuring that the study is conducted according to the protocol, ICH-GCP, regulatory requirements and standard operating procedures (SOPs).
The Clinical Research Associates also offers you the unique opportunity to have an exciting career in the research of drug and medical device development while making a difference in the lives of those around them.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Speak to our 24/7 chat and phone advisors to request information on partial scholarships and payment plans for nurses.
How much is a Clinical Research Coordinator’s salary?
Clinical research coordinators have the responsibility to oversee all daily activities of staff that are given the responsibility to conduct research. They have to do their work alongside principal investigators so as to make a determination of whether the study being conducted is viable or not. They also have to workout the budget at hand. They also need to make a review of all research protocols, making sure that all the test subjects know the protocols. They help in carrying out the research.
The Salary
When a clinical research coordinator is experienced, pay has quite an encouraging trend. If a clinicalresearch coordinator is a new entrant that has experience of less than five years, they earn a salary of around 43,000 dollars. This total is an average, according to salaries that were given by different anonymous users.
Usually, the compensation includes overtime payment, bonus payments, and even tips. In the mid of their careers, clinical research coordinators have experience of 5 to 10 years and they can expect a compensation of around 51,000 dollars. This is also an average that is based on different salaries submitted.
There is also the category of clinical research coordinators who have over 20 years’ experience in the field. For these professionals, the compensation is an average of 62,000 dollars and this is based on salaries submitted.
Clinical research coordinator salary by location
It is important to appreciate that different locations have different salary variations. There are some areas that pay more than the average, while others may pay less. If this is a career that you are willing to pursue, it is best that you find out as much as you can about the salary expected within your area. This information can help you negotiate for the best compensation for services rendered.
How to Become a CRA in Canada: Clinical Research Associate Certification
How to Become a CRA in Canada
How to Get A Clinical Research Associate Certificate Online in Canada
CCRPS offers Canadian CRA certification for students living and working in Canada. A CRA certification in Canada provides students with additional knowledge of resources and guidelines in order to work in clinical research.
Our certification specifically state and prove that you have reviewed Canadian guidelines in clinical research and meet the competencies required for all clinical research associates.While our certificate is accredited by the ACCRE, 4 other organisations, and shows employers you have the working knowledge to act as a CRA; the CRAC credentialing exam will help further establish your professionalism. If you already have experience as a clinical research associate, you can use this course to prepare and sit for the CRAC examination.
In order to become a certified Canadian CRA, you must take the CRAC credentialing examination. To sit for the examination, you must fulfill one of these requirements:
2 years of clinical research expereience within the last 5 years
3,500 hrs of part time experience with at least 1 year of Canadian experience within the last 5 years
A post-graduate certificate in clinical research and at least 1 years’ experience over the past 2 years in clinical research. You must have at least 1 year of Canadian experience.
2. Clinical Research Associate Canada (CRAC) Membership
While CCRPS membership is free, you still benefit from obtaining CRAC membership even if you do not have enough experience to sit for the CRAC credentialing exam. Membership can create networking and job opportunities, as well as provide insight into the field. All interested professionals should become a Member of the Official Canadian CRA Association and list this involvement on their CV and cover letter.
3. CCRPS Canadian CRA Certification (CCRAC) for Resume Building
As we teach nearly 30 Canadian students each quarter, our Canadian Clinical Research Associate Certification will allow students to strengthen their application and resume, specifically towards Canadian guidelines and policies.
4. Landing A Entry Level Clinical Research Associate Job in Canada
Unfortunately, many professionals have trouble securing a job in order to get experience and take the CRAC exam. Our certification, specifically created for Canadian CRAs, provides entry-level science professionals with all the resources they need to be a working CRA in Canada. Our online exam can be taken at any time, if you enroll or demo our course.
5. Clinical Research Associate Programs in Canada
In addition to providing 130 modules, our CRA certification exam is priced affordably and the same as our American students. Students can gain valuable work experience by applying to be one of our CCRPS Clinical Research Education Fellows for a 1 month remote internship to develop and strengthen your resume.
6. Salary of Canadian Clinical Research Associate
Payscale Canadian CRA Salary
Payscale Canadian Pay Difference CRA
Clinical Research Certification Specifically for Canadian CRAs
CCRPS offers one of the only online certifications specifically designed for entry-level and mid-level professionals in clinical research associate knowledge. We prepare you to understand and apply all skills required to secure a job in clinical research.
Canadian Clinical Research Associate Certificate Course Preview
Want to Learn More About Clinical Research?
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course