Clinical Research Certification Maryland: Everything You Need to Know for 2025-2026
Clinical research is one of the highest-leverage careers in Maryland’s booming biomedical and pharmaceutical economy. But simply entering the field isn’t enough anymore. Getting certified in clinical research isn’t just a “nice-to-have”—it’s the fastest way to multiply your salary, unlock better job roles, and become a top-choice candidate for global sponsors, CROs, and Maryland’s elite research hospitals like Johns Hopkins, NIH, and MedStar Health.
With the right certification, Maryland-based professionals can increase their salary by $15,000–$40,000 within 6–12 months, fast-track to roles like Clinical Research Associate (CRA), Clinical Trial Manager, or Regulatory Affairs Specialist, and gain eligibility for remote global trials. Whether you’re a nurse, pharmacist, public health grad, or data manager—clinical research certification makes you immediately employable in roles where unlicensed candidates get filtered out. Maryland employers are actively prioritizing certified professionals due to tighter FDA regulations and the increasing complexity of trial design.
What Is Clinical Research Certification in Maryland Exactly? Skills Required and Jobs Explained
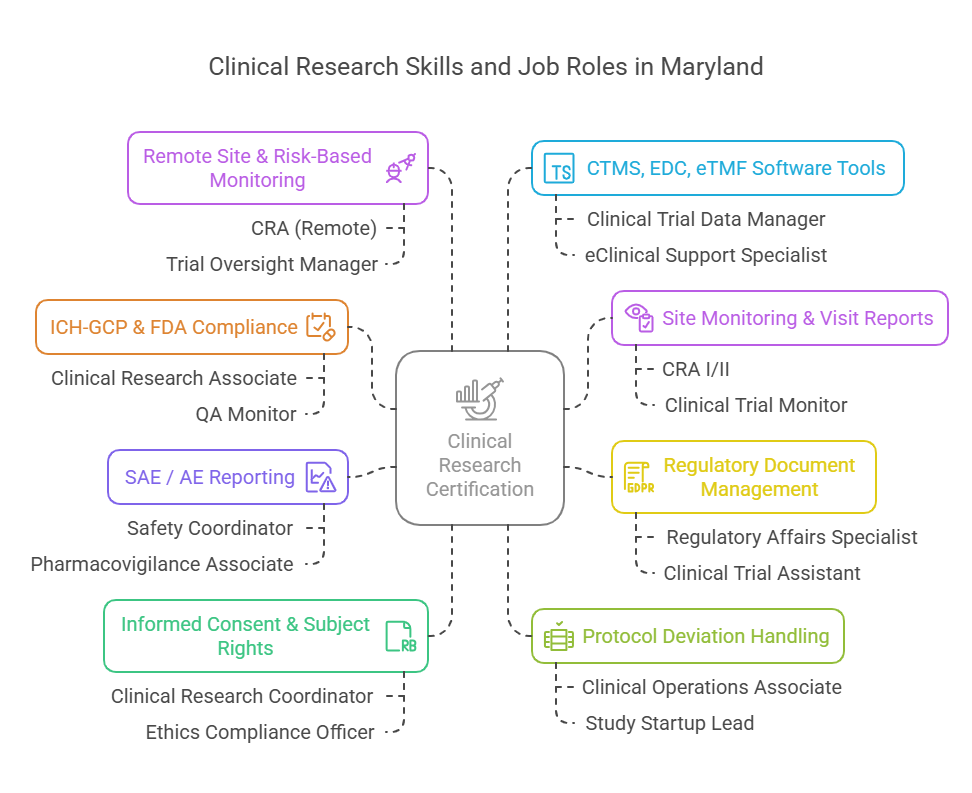
Clinical research certification in Maryland is an industry-recognized credential that proves you meet the global competency standards for designing, monitoring, and managing clinical trials. Whether issued by ACRP, SOCRA, or a globally accredited provider, this certification validates your ability to work on Phase I–IV clinical trials, comply with FDA and ICH-GCP guidelines, and perform protocol-specific responsibilities in regulated environments.
You’re not just learning theory. You're acquiring real job-ready skills—regulatory documentation, site management, adverse event tracking, data query resolution, subject recruitment, and protocol deviation handling—all of which are non-negotiables for roles at CROs, biotech firms, and Maryland’s top clinical trial institutions.
Why Should You Get Clinical Research Certification to Work in Maryland?
In Maryland’s clinical research market—where trial volume is high, oversight is strict, and employers are deeply tied to federal agencies like the NIH—having a certification isn’t just an edge; it’s a filter. If you apply to clinical trial roles at MedStar, Johns Hopkins, or federal contractors without one, your resume will likely get auto-rejected. Employers are actively prioritizing certified candidates to reduce audit risks and meet federal quality benchmarks.
With a certification, you’re not only seen as job-ready—you’re legally compliant, globally credible, and eligible for fast promotion cycles in trial monitoring, coordination, and regulatory support.
| Factor | With Clinical Research Certification | Without Certification |
|---|---|---|
| Job Role Access | CRA, CRC, Regulatory Associate, Safety Monitor, Trial Lead | Assistant, Recruiter, Data Entry, or Intern Roles |
| Average Salary (Maryland) | $74,000–$129,000+ annually | $42,000–$61,000 annually |
| Eligibility for Remote CRO Jobs | Yes — PPD, ICON, Medpace, Parexel actively hire | No — Filtered out by screening algorithms |
| Career Advancement | Fast-tracked to CRA II or Clinical Project Manager in 1–2 years | Stagnant growth, reliant on internal training (if available) |
| Global Job Portability | Meets EU, FDA, MHRA, TGA standards | Not recognized internationally |
| Trial Type Access | Phase I–IV, Oncology, Rare Disease, Device Trials | Limited to recruitment or observational studies |
Which Certification Should You Choose to Become a Clinical Research Professional in Maryland?
You’ll find dozens of certifications floating around: ACRP-CP, SOCRA-CCRP, CCRA, NIH’s GCP training, various university bootcamps. But the reality is, not all are designed equally. Some lack curriculum depth. Others don’t offer actual career services or practical tools.
For Maryland professionals aiming for high-paying roles at CROs (like ICON, PPD), sponsors (like AstraZeneca), or local research hospitals (like Johns Hopkins and MedStar), choosing a program with accreditation, depth, flexibility, and placement support is non-negotiable. That’s where CCRPS’s Clinical Research Certification stands apart.
| Feature | Typical Certifications | CCRPS's Clinical Research Coordinator Certification |
|---|---|---|
| Accreditation | Often limited to in-house or self-issued certificates | CPD-accredited, meets FDA/ICH-GCP standards |
| Curriculum Depth | Basic 5–10 module overview | 500+ modules covering CRA, CRC, SAE handling, EDC, audits |
| Learning Format | Only self-paced videos | Self-paced OR 4–12 week bootcamps (your choice) |
| Flexibility & Cost | No interest-free plans or lifetime access | Lifetime access + interest-free monthly payments |
| Instructor Access | Celebrity endorsements, no personal access | Direct access to industry experts & live coaching reviews |
| Transparency of Team | No visibility on who created the program | Built by ex-CRAs, CTMs, and regulatory specialists |
Why CCRPS’s Certification Will Be a Game Changer for Your Career in Maryland
The clinical research job market in Maryland is one of the most competitive in the U.S.—anchored by federal agencies, elite hospitals, and global CROs. But advancement in this space hinges on one thing: proven, certified expertise. That’s why CCRPS’s Clinical Research Certification is a strategic career move, not just a credential.
This certification equips you with job-ready clinical trial operations skills, real-world GCP application, and in-demand tech training (eTMF, CTMS, EDC tools). With 500+ modules and simulation-driven learning, you’ll walk into interviews with higher role eligibility, stronger salary leverage, and faster onboarding timelines.
More importantly, employers in Maryland—especially MedStar, NIH contractors, and CROs like ICON and Parexel—trust certified candidates to minimize compliance risk. CCRPS’s certification signals that you’re ready to manage FDA-regulated trials from Day 1.
Summarizing All You Need to Know About Getting Your Clinical Research Certification in Maryland
To help you quickly scan everything covered, here’s a summary of how CCRPS’s Clinical Research Certification compares to other paths in Maryland. Whether you're just entering the industry or pivoting from a clinical or regulatory background, this table clarifies why CCRPS is a dominant force in the East Coast research market.
| Key Factor | CCRPS Clinical Research Certification | Typical Alternatives |
|---|---|---|
| Accreditation | CPD-accredited, globally accepted, FDA-aligned | Limited, sometimes non-accredited |
| Curriculum Scope | 500+ modules, ICH-GCP, SAE, CRA/CRC job readiness | 5–10 modules, overview-level only |
| Maryland Employer Acceptance | Accepted by CROs, Johns Hopkins, NIH contractors | Often unknown or requires manual justification |
| Job Impact | $15K–$40K salary increase in 6–12 months | $0–$10K increase, often stagnant |
| Format | Self-paced + optional 4–12 week bootcamps | Self-paced only or rigid schedule |
| Support & Tools | 1-on-1 mentorship, toolkits, templates, lifetime access | Basic modules, no long-term support |
Frequently Asked Questions
-
Yes—especially if you're applying to roles at Johns Hopkins, NIH contractors, or CROs like ICON and PPD. While some entry-level roles may not require it, most employers use certification as a hard filter during screening. It proves you know GCP, FDA protocols, site monitoring, and compliance documentation. In Maryland’s competitive market, certified applicants are fast-tracked for interviews because employers want plug-and-play professionals who won’t need months of onboarding. Without certification, you’re often limited to administrative or intern-level positions. CCRPS’s certification opens doors to high-salary roles, global trials, and remote positions with CROs that require verified clinical ops knowledge.
-
Absolutely. CCRPS is CPD-accredited and globally recognized, with a curriculum aligned to FDA, ICH-GCP, and global trial standards. Employers in Maryland—including NIH-funded institutions, university hospitals, and biotech firms—acknowledge CCRPS as a valid, skills-first certification. Unlike academic programs that focus on theory, CCRPS is practical, job-focused, and built for direct workforce entry. The certification is accepted by remote CROs and on-site trial sponsors alike. Most importantly, it teaches Maryland-specific compliance nuances and equips you for both US-based and international trials. Hiring managers trust CCRPS-certified professionals because they’re easier to onboard and less likely to make regulatory errors.
-
SOCRA and ACRP offer strong certifications—but they often require prior experience, don’t include job training, and focus heavily on exams. In contrast, CCRPS offers over 500 practical lessons, templates, toolkits, and 1-on-1 mentorship, even for complete beginners. It also allows self-paced learning or 4–12 week bootcamp options. In Maryland’s real-world job market, employers care more about your ability to manage eTMFs, handle protocol deviations, and communicate with sponsors than just passing an exam. CCRPS prepares you for the actual role, not just the test. It’s also faster to complete and more affordable, with lifetime access and payment plans.
-
It depends on your pace. Most students complete CCRPS’s certification in 4 to 12 weeks, especially those choosing the guided bootcamp path. If you're balancing work or family, the program is 100% self-paced—so you can go slower without losing access. The platform is designed for professionals with zero background in clinical trials, making it suitable for nurses, pharmacists, lab techs, or public health grads pivoting into research. You can start immediately after enrolling, and there’s no deadline, no exam pressure, and no expiration on your certification. Many Maryland students complete it over evenings or weekends while working full-time.
-
Yes. CCRPS includes career support resources, LinkedIn optimization tips, resume templates, and mock interview walkthroughs specific to clinical research. While it’s not a placement agency, the course includes modules on how to apply to jobs at CROs like Parexel, Medpace, ICON, and Maryland trial sites. You’ll also learn how to highlight your certification when applying to NIH, FDA contractors, or academic medical centers. Many learners secure jobs within 3–6 months post-certification. More importantly, CCRPS’s reputation boosts your profile on application tracking systems, ensuring your resume gets seen by recruiters screening for certified applicants.