The Ultimate Guide to Getting Medical Science Liaison & Monitor Certification in UK: Everything You Need to Know in 2025
In the UK’s rapidly expanding clinical research and medical affairs sector, becoming a certified Medical Science Liaison (MSL) or Medical Monitor isn’t optional anymore—it’s strategic. NHS-academic partnerships, post-Brexit regulatory shifts, and decentralised trials have raised the bar. Employers no longer just want skilled professionals; they want certified, audit-ready experts trained in both real-time trial safety and high-level stakeholder engagement. If you're aiming for sponsor-facing roles, safety oversight, or direct involvement with MHRA and EMA submissions, this certification is your baseline credential.
More importantly, it’s also your salary lever. Recent job placement trends across London, Cambridge, and Manchester show that certified MSLs and Medical Monitors earn 28%–45% more than uncertified peers. The difference isn’t just pay—it's access to hybrid roles, global sponsors, and regulatory influence. This guide unpacks exactly what the certification involves, how to get it, and how to make it a fast-track upgrade to your clinical career.
What Is Medical Science Liaison & Monitor Certification in the UK Exactly? Skills Required and Jobs Explained
A Medical Science Liaison & Monitor Certification in the UK validates your capability to operate at the intersection of clinical safety oversight and scientific stakeholder engagement. It equips professionals to confidently interact with regulators like the MHRA, lead trial risk decisions, and build long-term influence with KOLs across NHS Trusts and commercial research sponsors. This dual-role training is especially vital in a post-Brexit environment where UK-based certification and documentation compliance are now being treated separately from EU tracks, and employers need individuals fluent in both GCP and strategic medical communications.
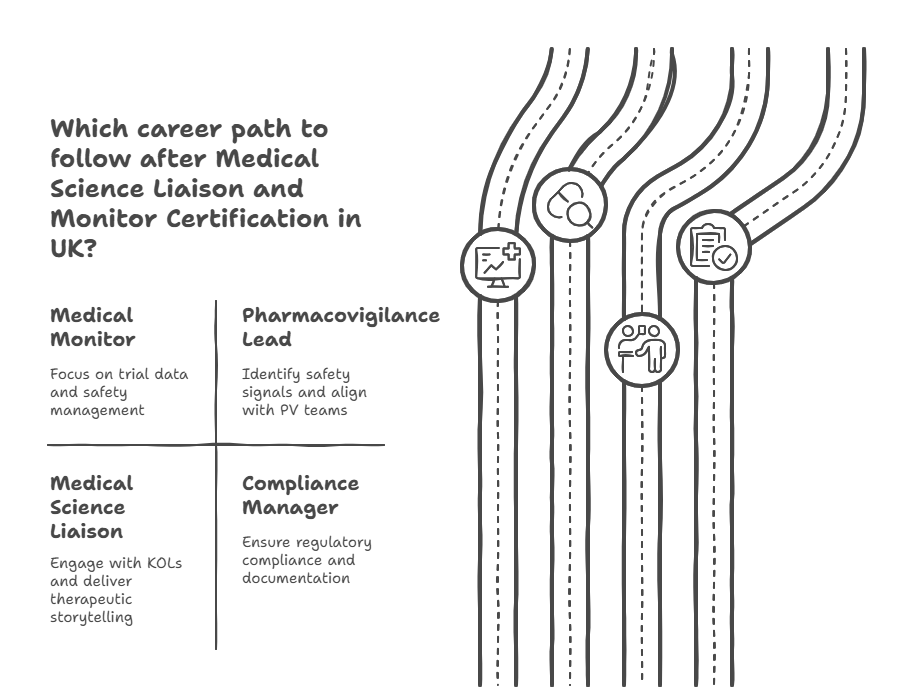
What Skills Does a Certified Medical Monitor in UK Actually Gain?
Monitor clinical data in real time, identify AE/SAE patterns, and lead safety escalation with MHRA alignment.
Detect and act on medical risk signals, coordinate with PV teams, and write escalation briefings.
Engage KOLs professionally, present therapeutic data, and prepare for advisory board participation.
Write and review regulatory documentation that complies with UK-specific requirements, GCP, and trial legislation.
Deliver scientific insights and strategy, including storytelling, label interpretation, and evidence communication.
Generate trial-critical documentation, including MSL slide decks, site visit reports, CRM entries, and follow-up summaries.
What Jobs Can You Apply for With Medical Monitor Certification in UK?
Medical Monitor (IQVIA, ICON, Parexel)
Clinical Safety Physician (GSK, AstraZeneca, PPD)
Pharmacovigilance Lead (Pharmalex, Labcorp, Syneos Health)
Medical Science Liaison (MSL) (Pfizer, Novartis, Sanofi)
Medical Affairs Officer (Boehringer Ingelheim, UCB, Amgen)
Scientific Communications Specialist (Oxford PharmaGenesis, Fishawack Health, Prime Global)
Regulatory Strategy Advisor (MHRA contractors, PRA Health Sciences, Thermo Fisher Scientific)
Why Should You Get a Medical Science Liaison & Monitor Certification to Work in the UK?
In the UK’s post-Brexit research economy, clinical employers increasingly prioritise certified professionals who can work across both trial safety and stakeholder strategy. Without a formal Medical Science Liaison & Monitor Certification, most applicants are locked into support roles—excluded from key medical decision-making, regulatory responses, and sponsor communication. With this dual-role certification, you’re instantly positioned for senior-facing roles at global CROs, pharma giants, and NHS-affiliated research hubs. It’s not just a credential—it’s the minimum trust signal MHRA-facing employers want when hiring for high-accountability trial functions.
| Career Factor | Without Certification | With Certification |
|---|---|---|
| Role Eligibility | Limited to support roles like PV assistant or junior CRA | Eligible for MSL, Medical Monitor, and Medical Affairs Lead roles |
| Regulatory Involvement | Excluded from safety escalation or MHRA submissions | Directly involved in documentation, DSURs, and protocol responses |
| Average Salary Range | £38,000–£52,000 annually | £65,000–£92,000+ annually |
| Job Mobility (UK/EU) | Restricted to site-level roles with low upward potential | Eligible for UK-wide, hybrid, and sponsor-track roles |
| Recruiter Visibility | Overlooked in CRO and sponsor filtering systems | Prioritised in algorithmic hiring filters and internal referrals |
Which Certification Should You Choose to Become a Medical Monitor or MSL in the UK?
Not all certifications are created equal. In the UK, many professionals default to fragmented options like short PV courses or isolated MSL slide training on Coursera, DIA, or LinkedIn Learning. While these offer surface-level exposure, they lack depth, regulatory compliance alignment, and most critically—real-world training that employers now expect. Few of them cover both medical monitoring and scientific engagement in a single track, and even fewer provide simulations, MHRA-focused compliance, or live mentorship from industry experts.
That’s where CCRPS’s Medical Science Liaison & Monitor Certification stands out. It’s a dual-role program designed for MDs, PhDs, PharmDs, and MSc professionals working in the UK or remote EU sponsor settings. With 249+ modules, CPD + CME accreditation, live casework, optional bootcamps, and certification that’s instantly verifiable—CCRPS prepares you for trial oversight and sponsor dialogue from day one. No fluff, no gatekeeping, no celebrity instructors—just actual industry experts and job-relevant assets.
| Feature | Typical Programs | CCRPS Medical Science Liaison & Monitor Certification |
|---|---|---|
| Accreditation | No CPD or UK-recognised CME credits | CPD and CME accredited, valid UK-wide with verifiable digital certificate |
| Curriculum Depth | Slides or PDF-based content, no casework | 249+ modules covering AE/SAE triage, label strategy, GCP, KOL engagement |
| Training Format | Pre-recorded, no interaction or assessment | Simulation-based, live webinars, project deliverables, lifetime access |
| Instructor Access | No support, anonymous trainers, or celebrities | Real industry instructors, transparent team, live review options |
| Flexibility & Payments | One-time access, no upgrades, no plans | Self-paced or bootcamp, with interest-free monthly payment plans |
| Career Support | None, or generic downloadable CV tips | Optional mentorship, job prep, mock interviews, and recruiter coaching |
Why CCRPS Medical Science Liaison & Monitor Certification Will Be a Game Changer for Your Career in the UK
The UK’s life sciences sector is projected to grow by over £30 billion by 2030, and MHRA-aligned roles in medical monitoring and liaison strategy are at the center of that expansion. But employers aren’t just hiring titles—they’re hiring certified competencies. The CCRPS Medical Science Liaison & Monitor Certification gives professionals the rare ability to bridge both trial safety and scientific communications. That combination is now crucial for sponsor-track roles, hybrid MSL deployment, and site-risk leadership in NHS trials and decentralized models.
Across London, Oxford, Manchester, and remote UK-based clinical research teams, professionals who’ve completed the CCRPS program have reported salary increases of 22% to 41% within the first 9 months. These are based on placement data from 2023–2025 hires into roles like Medical Monitor, MSL, Medical Affairs Lead, and PV Manager, across both CROs and pharma sponsor organizations. It’s a transformation in both earnings and job tier.
Summarizing All You Need to Know About Getting Your CCRPS Medical Science Liaison & Monitor Certification in the UK
If you're serious about stepping into high-trust clinical or medical affairs roles in the UK, this certification gives you both the skills and credibility that hiring managers at MHRA-regulated sponsors, CROs, and biotech firms are actively filtering for. The table below captures everything you need to know before enrolling in the CCRPS Medical Science Liaison & Monitor Certification—from eligibility to career outcomes, accreditation to salary impact.
| Category | Details |
|---|---|
| Certification Name | CCRPS Medical Science Liaison & Monitor Certification |
| Eligibility | MDs, PhDs, PharmDs, MSc holders in life sciences, CRAs, PV professionals |
| Accreditation | CPD & CME-accredited, MHRA and GCP-aligned, recognised UK-wide |
| Learning Format | 249+ modules, live webinars, simulations, lifetime access |
| Training Tracks | Self-paced learning or 4–16 week bootcamp options with mentorship |
| Skills Gained | Safety monitoring, KOL engagement, MHRA compliance, trial documentation |
| Job Titles | MSL, Medical Monitor, PV Manager, Medical Affairs Officer, Safety Reviewer |
| Employers Hiring in UK | Pfizer, IQVIA, AstraZeneca, UCB, Labcorp, MHRA contractors |
| Expected Salary Increase | 22%–41% within 6–12 months post-certification |
| Recognition Scope | Valid across UK sponsors, NHS trials, hybrid remote CRO teams |
Frequently Asked Questions
-
Yes. The CCRPS certification is CPD and CME-accredited, which aligns with professional development standards recognized across the UK healthcare and clinical research sectors. It covers UK-specific compliance topics like MHRA regulations, trial monitoring expectations under UK Clinical Trials Regulation, and scientific engagement practices consistent with ABPI guidelines. Whether you’re applying to roles at NHS-affiliated academic centres, global CROs with UK hubs, or sponsor-facing biotech companies, this certification is accepted and respected. It’s already helped professionals get hired at IQVIA, AstraZeneca, UCB, and Pfizer UK.
-
Most professionals finish the program in 4 to 8 weeks part-time, or as quickly as 2–3 weeks full-time. A fast-track bootcamp option is available for those who want structured, instructor-guided acceleration. Because you get lifetime access, there's no pressure to rush. The curriculum is simulation-based, meaning you’ll complete real-world deliverables (like MSL slide decks, AE narratives, visit reports), not just multiple-choice quizzes. This makes it job-ready, not just paper-certified. Many learners report job interviews and recruiter interest within weeks of completing the final module.
-
The certification is highly relevant to CROs, biotechs, academic research hospitals, and pharma sponsors operating in the UK. This includes companies like Parexel, Syneos, ICON, IQVIA, and sponsor-facing roles at Pfizer UK, Novartis, AstraZeneca, and UCB Pharma. NHS-affiliated research centres also value candidates who are GCP-certified and trained in scientific stakeholder communication and medical oversight—the exact focus of this program. Recruiters increasingly use certification filters when sourcing candidates, especially for hybrid MSL, PV, and trial strategy roles.
-
You’ll complete MSL slide decks, clinical monitoring reports, stakeholder engagement plans, SAE escalation summaries, and scientific exchange documentation. One module simulates a full KOL engagement lifecycle—from pre-call planning to post-call CRM documentation. Another walks you through responding to a mock MHRA regulatory query. These projects are reviewable, reusable, and tailored for real job interviews or role readiness. Instead of learning theory, you're building assets you can show in hiring conversations at CROs or sponsor organisations.
-
Support includes 1-on-1 mentorship options, live Q&A webinars, and job readiness coaching (CV review, mock interviews, role targeting). While the core course is self-paced, many UK learners opt for the bootcamp add-on, which offers structured weekly guidance and instructor feedback. After certification, CCRPS provides alumni with continued access to updated modules and recruiter outreach support. It's more than a one-time course—it's a full career support pipeline, especially valuable when applying to sponsor-level UK roles.