Clinical Research Coordinator Career Growth: How to Get Promoted?
Advancing in your career as a Clinical Research Coordinator (CRC) requires a strategic approach that encompasses understanding the career ladder, obtaining additional certifications, and distinguishing yourself in the field. This comprehensive guide will delve into these aspects, providing you with actionable insights to facilitate your professional growth.
Understanding the Career Ladder for Clinical Research Coordinators
The career progression for CRCs typically follows a structured path, allowing for advancement through various roles:
Clinical Research Assistant: An entry-level position focusing on basic research support tasks.
Clinical Research Technician: Involves more technical responsibilities, including data collection and patient interaction.
Clinical Research Coordinator Associate: Manages specific aspects of clinical trials under supervision.
Clinical Research Coordinator Intermediate: Takes on greater responsibility, overseeing multiple studies and ensuring compliance with protocols.
Clinical Research Coordinator Senior: Leads complex trials, mentors junior staff, and contributes to protocol development.
Clinical Research Coordinator Lead: Supervises a team of coordinators, manages resources, and ensures the success of multiple studies.
Clinical Research Project Manager: Oversees the planning, execution, and completion of clinical research projects.
Pursuing Additional Certifications
Certifications can significantly enhance your credibility and open doors to higher-level positions. Consider the following:
Certified Clinical Research Coordinator (CCRC): Offered by the Association of Clinical Research Professionals (ACRP), this certification validates your expertise in clinical research coordination.
Certified Clinical Research Professional (CCRP): Provided by the Society of Clinical Research Associates (SOCRA), this credential demonstrates proficiency in clinical research regulations and practices.
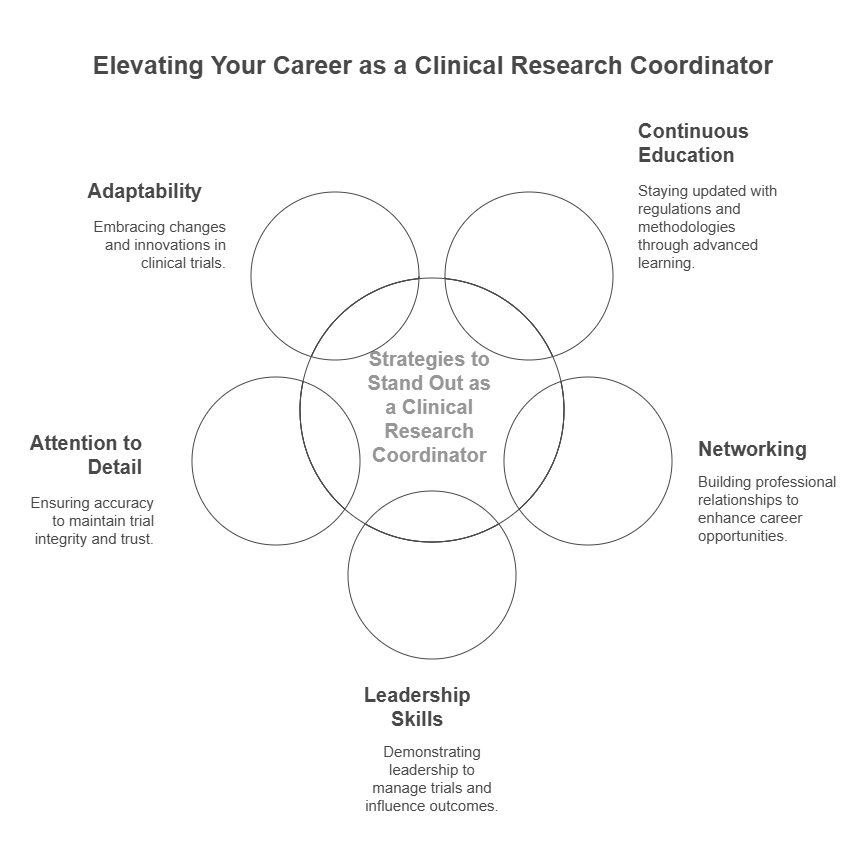
Strategies to Stand Out in the Field
The clinical research industry's growing competition and speed require Clinical Research Coordinators to move past basic responsibilities for professional distinction. The workforce now demands employees who deliver additional value through their leadership abilities and adaptability and proactive learning approaches. Below are the most impactful strategies to build a reputation as a high-performing CRC in 2025 and beyond:
1. Continuous Education
The clinical research field is highly regulated and constantly evolving due to scientific advancements, technology adoption, and shifting compliance requirements. It is important to stay informed in order to be up to date with Good Clinical Practice (GCP), FDA regulations and new trial methodologies.
How to do it:
Enroll in specialized training like ICH-GCP certification, Good Documentation Practices, or Clinical Data Management.
Pursue advanced degrees such as a Master’s in Clinical Research, Public Health, or Pharmaceutical Sciences.
Take accredited online courses offered by CCRPS, Coursera, ACRP, and SOCRA.
Stay current with peer-reviewed journals like Applied Clinical Trials and The Lancet.
2. Networking
Professional visibility and career opportunities along with collaborations and mentorship emerge from networking activities. The people you know in clinical research enable you to obtain access to new protocols and promotions and leadership positions.
How to do it:
Join professional associations like SOCRA, ACRP, and DIA (Drug Information Association).
Attend industry-specific conferences such as MAGI Clinical Research Conference or ACRP’s Annual Meeting.
Engage on LinkedIn by contributing to CRC-related groups and discussions.
Find a mentor within your organization or through industry meetups who can guide your career.
3. Leadership Skills
The coordination of clinical trials requires interaction among sponsors, investigators, IRBs, and patients. Leadership is important for a CRC to manage timelines, resolve issues, and influence outcomes, all of which are important for promotions.
How to do it:
Volunteer to lead new trial startups or protocol amendments.
Take ownership of training junior CRCs or new team members.
Get certified in project management or clinical trial management.
Show initiative by suggesting workflow improvements or digital tools that enhance trial efficiency.
Lead patient recruitment efforts or data integrity initiatives.
4. Attention to Detail
Clinical research operates on data-based principles. A single incorrect entry in documentation or data entry process may lead to protocol deviation, regulatory warning or loss of trial integrity. Attention to detail helps to gain trust from supervisors and sponsors.
How to do it:
Double-check source documentation and electronic data capture (EDC) entries.
Maintain audit-ready records at all times.
Learn the nuances of protocol compliance, adverse event reporting, and informed consent documentation.
Utilize checklists, trackers, or apps like RedCap or Clinical Conductor to manage tasks efficiently.
Attend refresher training on documentation best practices.
5. Adaptability
The clinical trials landscape is changing quickly with the growth of decentralized clinical trials (DCTs), remote monitoring, and AI-powered data systems. You are an asset during transitions and innovative protocol changes when you are adaptable.
How to do it:
Get comfortable with using digital tools such as eSource platforms, eConsent tools, and wearable device data integration.
Stay updated with regulatory trends, like FDA’s digital health guidance or EMA’s transparency rules.
Learn to work across hybrid trial environments, balancing on-site and remote responsibilities.
Embrace training in telehealth platforms, virtual visits, or patient-centric trial models.
Lesser-Known Facts About Clinical Research Coordination
Global Demand: The need for skilled Clinical Research Coordinators (CRCs) is rising worldwide, offering opportunities for international career advancement. This demand is driven by the expanding pharmaceutical and biotechnology industries globally. (Source)
Technological Integration: Emerging technologies like artificial intelligence (AI) are being integrated into clinical trials, requiring CRCs to adapt and learn new skills. AI is expected to enhance efficiencies and improve outcomes in clinical research. (Source)
Regulatory Variations: Different countries have unique regulatory requirements for clinical trials, making knowledge of international guidelines beneficial. Understanding these variations is crucial for ensuring compliance in global clinical trials.
Patient Advocacy: CRCs often play a role in advocating for patient rights and ensuring ethical standards are upheld. They manage the conflict between research and care, ensuring individualized care within the context of research. (Source)
Remote Monitoring: The rise of decentralized trials has led to an increase in remote monitoring responsibilities for CRCs. This shift requires adaptability and technological proficiency. (Source)
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
Climbing the career ladder as a Clinical Research Coordinator requires more than just meeting daily responsibilities. It takes a blend of continuous learning, strong leadership, attention to detail, adaptability, and meaningful networking. By committing to these strategies, you not only position yourself for promotion but also become a trusted and invaluable asset in any clinical research team. For professionals seeking structured training and certification, CCRPS offers industry-recognized programs that support long-term growth and advancement in clinical research.
Frequently Asked Questions (FAQs)
-
A bachelor's degree in health sciences, nursing, biology, or a related field is typically required. Some positions may prefer or require a master's degree.
-
The timeline varies depending on the certification body and your prior experience, but generally, it involves meeting educational and professional experience requirements, followed by passing a certification exam.
-
Yes, with the rise of decentralized and virtual clinical trials, remote work opportunities for CRCs have increased.
-
The demand for CRCs is expected to grow due to the increasing number of clinical trials and the need for skilled professionals to manage them.
-
Yes, CRCs can advance to roles such as Clinical Research Associate, Clinical Trial Manager, or Regulatory Affairs Specialist, among others.