Common Challenges in Clinical Research and How to Overcome Them?
Clinical research is a cornerstone of medical progress, yet conducting trials comes with a range of obstacles. From regulatory complexities to patient recruitment struggles and data integrity concerns, the path to successful clinical trials is often rocky. This blog explores the most common challenges in clinical research and provides actionable strategies to overcome them in 2025 and beyond.
1. Regulatory Hurdles in Clinical Research
The process of navigating clinical research regulations stands as one of the most complicated undertakings. The FDA (Food and Drug Administration), EMA (European Medicines Agency), and ICH-GCP (International Council for Harmonisation – Good Clinical Practice) establish detailed regulations to guarantee safe and ethical execution of clinical trials. The constantly changing nature of these standards leads to regulatory delays and increased complexity in trial processes.
Key Regulatory Barriers:
Lengthy Approval Timelines: The process of obtaining clinical trial approval extends beyond what researchers anticipate. The regulatory process requires extra documentation from agencies which causes delays in the approval timeline. The ongoing process of compliance monitoring continues after receiving approval.
Inconsistent Global Requirements: The different regulatory requirements across regions create challenges for implementing standardized processes in international trials. What is acceptable in one country may not be in another.
Changing Documentation Protocols: Regulations may change over time and documentation must be updated to reflect these changes. This constant change can lead to confusion and potential errors.
Solutions:
Hire Experienced Regulatory Affairs Professionals: The professionals have a good understanding of the needs of various regulatory bodies and can thus expedite the approval process by making sure that the submissions are proper and well arranged.
Use Centralized IRB/Ethics Committees: The Institutional Review Boards (IRBs) and Ethics Committees protect the safety and rights of participants. The centralization of these reviews can save time and prevent delays.
Leverage AI-Powered Regulatory Compliance Tools: AI tools can help track regulatory changes and ensure compliance by automating documentation, which can reduce human error and speed up approvals.
2. Patient Recruitment and Retention Issues
Recruitment and retention of patients is a critical challenge that almost 80% of clinical trials face, particularly those involving complex medical conditions or rare diseases. The inability to recruit enough patients can lead to trial delays or even cancellations.
Barriers Include:
Narrow Eligibility Criteria: Strict inclusion/exclusion criteria often limit the pool of eligible patients, making it difficult to recruit the necessary number of participants.
Lack of Patient Awareness: Many patients are simply unaware of clinical trials available to them, or they may not understand the benefits or risks involved.
Language and Cultural Gaps: Particularly in global trials, language barriers and cultural differences may prevent participation from diverse populations.
Solutions:
Use Real-World Data (RWD) for Better Targeting: By using RWD, researchers can identify potential candidates who meet specific criteria, improving recruitment efficiency.
Partner with Patient Advocacy Groups: These groups can help raise awareness and build trust within patient communities, which is essential for recruitment.
Provide Flexible Visit Scheduling and Travel Support: Flexible scheduling and providing travel reimbursement or arrangements can help ensure patient retention and minimize drop-off rates.
3. Data Management Challenges
Effective data management is essential for the success of any clinical trial. Inaccurate, incomplete, or inconsistent data can lead to incorrect conclusions or regulatory rejections. Managing large datasets from multiple sites, particularly when trials are global, adds further complexity.
Common Problems:
Inconsistent Data Entry: Inconsistencies in how data is entered, particularly when researchers at multiple sites are involved, can cause discrepancies.
Lack of Standardized Formats: Without standardized data formats, comparing or aggregating data from different sites becomes challenging.
Cybersecurity Threats: Protecting sensitive patient data is a top priority, and any data breach can result in serious legal and reputational consequences.
Solutions:
Implement Secure EDC (Electronic Data Capture) Platforms: EDC systems automate the collection and storage of clinical trial data, ensuring data integrity and compliance with regulatory requirements.
Train Sites Thoroughly on SOPs: Standard Operating Procedures (SOPs) ensure that data entry and handling are consistent across all trial sites.
Conduct Real-Time Data Monitoring and Auditing: Real-time monitoring allows researchers to detect and correct errors as soon as they arise, improving data accuracy and quality.
4. Funding and Budget Constraints
Clinical trials are expensive endeavors, with costs that can range from $2 million to $20 million depending on the phase and complexity of the trial. Budget overruns are common, and many trials are halted due to insufficient funding.
Challenges:
Unexpected Operational Costs: Hidden costs such as additional site visits, changes in trial protocols, or unanticipated regulatory fees can throw off the budget.
Underestimated Recruitment Costs: The expense of recruiting patients, especially when using incentives or travel reimbursements, often exceeds initial estimates.
Delays Leading to Overspending: Delays in recruitment or regulatory approvals can extend trial timelines, increasing overall costs.
Solutions:
Conduct Robust Feasibility Studies: Proper feasibility assessments before launching a trial help identify potential cost overruns early on and refine the budget.
Secure Diversified Funding (Grants, CRO Partners, Investors): By securing funding from multiple sources (grants, CROs, private investors), you ensure financial stability and mitigate risk.
Use Predictive Budgeting Tools: Predictive analytics can forecast the financial needs of the trial more accurately, helping manage costs and resources.
5. Ethical Considerations
Ensuring that clinical research meets ethical standards is fundamental to maintaining public trust and participant safety.
Challenges:
Informed Consent Clarity: Patients must understand the risks and benefits of participating in a trial. Complex medical terminology can lead to misunderstandings.
Risk-Benefit Balance: Ethical trials must weigh the potential risks to participants against the benefits of the research.
Protecting Vulnerable Populations: Certain groups (minors, elderly, pregnant women) are considered vulnerable and require extra protections in clinical research.
Solutions:
Use Plain-Language Consent Forms: Consent forms should be written in clear, easy-to-understand language to ensure that participants fully understand what they are agreeing to.
Apply Ethical AI Tools in Trial Design: AI can be used to design more ethical trials by assessing potential risks and balancing them against the scientific value.
Conduct Ethics Training for Investigators: Training ensures that all clinical trial staff are aware of their ethical responsibilities and understand how to address potential ethical issues.
6. Site Selection and Coordination
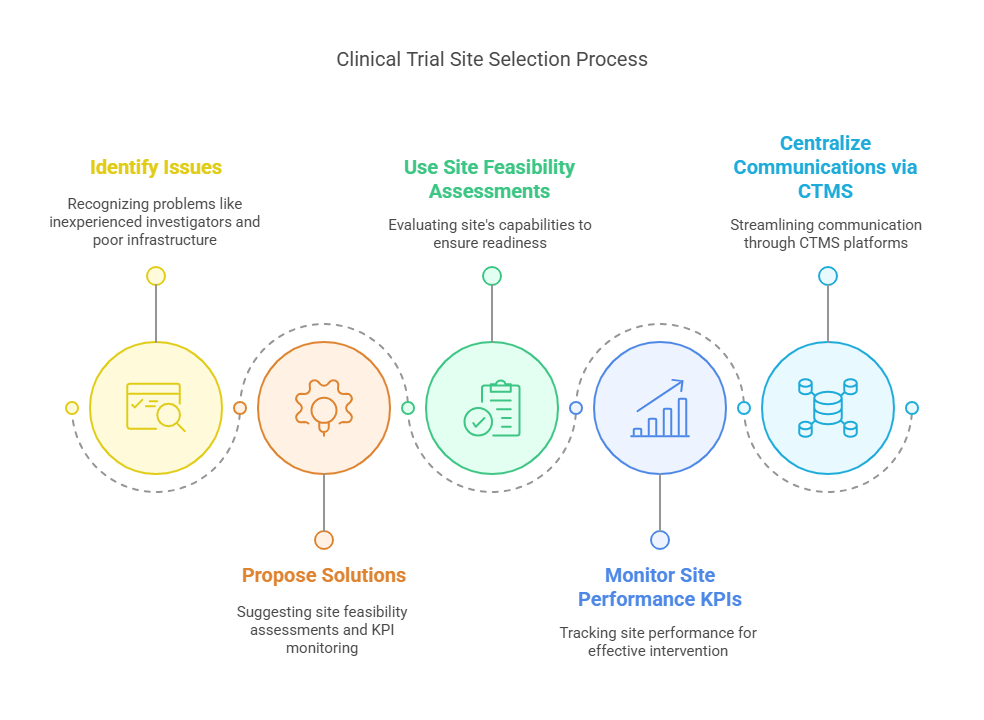
The selection of clinical trial sites plays a significant role in determining the success or failure of a study. Poor site selection can lead to delays, data quality issues, and even the abandonment of a trial.
Issues:
Inexperienced Investigators: If the site staff lacks experience or training in clinical research, they may fail to meet study requirements or collect accurate data.
Inadequate Infrastructure: Sites may not have the necessary resources or equipment to conduct the trial properly.
Poor Communication: Miscommunication between trial sponsors and investigators can lead to misunderstandings, delays, and errors.
Solutions:
Use Site Feasibility Assessments: These assessments evaluate the site's capabilities, ensuring that it is adequately prepared to handle the trial’s requirements.
Monitor Site Performance KPIs: Key performance indicators (KPIs) track the effectiveness and efficiency of trial sites, allowing sponsors to intervene when necessary.
Centralize Communications via CTMS Platforms: Clinical Trial Management Systems (CTMS) streamline communication, ensuring that everyone is on the same page and that tasks are completed on schedule.
7. Lack of Diversity in Clinical Trials
Clinical trials are often criticized for underrepresenting minority populations. This lack of diversity can skew results and make it difficult to apply findings universally.
Solutions:
Community-Based Trial Sites: By conducting trials in diverse communities, researchers can better represent different populations.
Multilingual Outreach Programs: These programs make it easier for non-English-speaking populations to participate in clinical trials.
Inclusive Eligibility Criteria: Trial inclusion criteria should be broad enough to include a diverse range of participants.
8. Technology Adoption Resistance
Many stakeholders in clinical research are reluctant to adopt new technologies due to concerns over cost, training, and the complexities of integrating these tools into existing systems.
Barriers:
Training Gaps: Not all staff may be trained to use new technologies effectively.
Cost Concerns: Implementing new tools often requires significant investment, which can be a deterrent.
Fear of Change: Resistance to change can result in reluctance to adopt digital solutions like ePRO (electronic patient-reported outcomes) or wearables.
Solutions:
Gradual Tech Integration: Introduce new tools incrementally to ease the transition and allow time for staff training.
Vendor-Provided Training: Many tech vendors offer training programs to ensure that users are proficient with new systems.
Stakeholder Buy-In Sessions: Engaging stakeholders early on and showcasing the long-term benefits of technology can help overcome resistance.
9. Global Trial Complexities
When conducting multi-country trials, a variety of factors—including cultural differences, regulatory requirements, and logistics—can create significant challenges.
Issues:
Import/Export Regulations: Trials may require the movement of medical products between countries, which can be delayed by customs regulations.
Language Translation: Ensuring that all documentation, consent forms, and communications are accurately translated is essential to avoid misunderstandings.
Local IRB Delays: Different countries may have varying timelines for Institutional Review Board (IRB) approval, leading to delays in starting the trial.
Solutions:
Hire Global CROs: Global Contract Research Organizations (CROs) have experience managing international trials and navigating the associated complexities.
Use Bilingual Documentation: Providing documentation in the local language ensures better understanding and compliance.
Plan Country-Specific Timelines: Factor in regional delays when planning the trial’s overall timeline to avoid unnecessary setbacks.
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
Navigating the complex world of clinical research requires a partner who understands the challenges and has proven tools to solve them. CCRPS (Certified Clinical Research Professionals Society) offers advanced training, global certification programs, and expert resources tailored to today’s research professionals. Whether you're aiming to improve recruitment, master regulatory compliance, or enhance data quality, CCRPS can guide your journey to trial success.
Frequently Asked Questions (FAQs)
-
Patient recruitment continues to be the top challenge, often leading to trial delays or cancellations.
-
2025 sees more decentralized trials, AI-powered tools, and patient-centered designs.
-
Clean, accurate data ensures trial validity and regulatory approval.
-
By setting up community-based sites, using inclusive language, and engaging underserved populations early.