Clinical Research Sites Directory for Rare Diseases Studies
Rare diseases affect over 300 million people globally, yet fewer than 5% of these conditions have FDA-approved treatments. The gap between diagnosis and therapeutic discovery is often widened by a lack of trial-ready research sites with rare disease specialization. Clinical Trials Sponsors face major bottlenecks in site identification, especially for first-in-human studies with genetically defined subgroups. Recruitment can stall for months, not due to patient scarcity—but due to the absence of capable infrastructures, validated diagnostic tools, and teams experienced in rare disease trials. The complexity of these protocols requires deep site-level competency and cross-border collaboration from day one.
As rare disease pipelines expand—with orphan drug designations rising over 300% in the last decade—the global demand for highly specialized clinical trial sites has intensified. But these aren’t plug-and-play locations. They require pediatric or geriatric capability, access to biorepositories, and precision diagnostic support. Most importantly, they need staff trained in genetic counseling, patient advocacy engagement, and rare disease registry coordination. This directory aims to map out the top-performing sites worldwide—those that deliver not only operational readiness, but also the ethical and scientific nuance needed for rare disease research.
Why Rare Disease Trials Require Specialized Research Sites
Complex Protocols and Smaller Populations
Rare disease trials operate under unique constraints that general research sites are rarely prepared for. These studies often involve fewer than 200 total global participants, making every site’s enrollment performance critical to the study’s survival. Protocols are typically adaptive, biomarker-driven, and designed around specific mutations, often requiring additional ethical clearances and pre-enrollment diagnostic steps. This increases screening burden, prolongs the time to first patient-in, and limits who can participate.
Sites must be equipped with advanced diagnostic labs, rare variant genotyping capabilities, and access to existing rare disease biobanks. Even consenting procedures require added sensitivity—especially in pediatric cases or when genetic risk disclosures are involved. This complexity translates into significant delays at generalist institutions. In contrast, specialized rare disease sites have pre-built SOPs, integrated molecular teams, and IRBs familiar with orphan protocols. Their experience with low-N studies also ensures that statistical power and protocol compliance remain intact throughout trial conduct.
Patient-Centric Models and Genetic Focus
Rare disease trials demand longitudinal engagement, often spanning several years, with patients who may face mobility, cognitive, or systemic health challenges. Research sites need to pivot away from traditional high-throughput models and instead build relationship-based frameworks that center family support, multi-specialist integration, and telemedicine continuity.
What separates specialized sites is their use of patient-centric trial design from the outset. They work closely with advocacy groups and patient registries during protocol development to ensure inclusion criteria reflect the lived reality of those affected. Many also co-develop protocols with families, integrate electronic health records (EHRs) into trial workflows, and support remote data collection for patients unable to travel.
Equally critical is the genetic infrastructure. Trials may rely on rare variant identification, whole-exome sequencing, or pharmacogenomic matching. Specialized sites have in-house genomic counselors or direct access to geneticists, accelerating time from referral to eligibility confirmation. This blend of genetic readiness and relational care is what enables consistent enrollment, lower dropout rates, and regulatory-ready data quality.
Site Capabilities That Enable Rare Disease Studies
Biomarker Analysis and Genomic Sequencing
For rare disease trials, eligibility and efficacy are often contingent on biomarker presence or specific gene mutations. Sites must therefore go beyond standard diagnostics. They require infrastructure for next-generation sequencing (NGS), qPCR panels, and high-throughput omics platforms. Without this, delays in patient stratification can derail study timelines.
Specialized sites typically integrate lab capabilities either in-house or through immediate partnerships with accredited molecular labs. This ensures rapid turnaround on variant interpretation, enrollment decisions, and ongoing pharmacodynamic assessments. Sites must also be equipped to handle biological specimen logistics, including dry ice shipping, global biobank compliance, and re-consent protocols for future genetic research. These capabilities are non-negotiable for sponsors running trials in rare oncology, neurometabolic, or immunodeficiency domains.
Pediatric and Geriatric Specialization
Over 50% of rare diseases manifest in childhood, while others—like idiopathic pulmonary fibrosis or amyloidosis—affect geriatric populations. This dual spectrum requires research sites to have both pediatric research infrastructure and geriatric clinical accommodations, including age-appropriate dosing, mobility support, and adaptive communication protocols.
Pediatric sites must adhere to strict assent/consent standards, have access to child psychologists, and use child-sized medical devices for imaging or biospecimen collection. For geriatric patients, considerations around polypharmacy, comorbidity risk, and functional capacity are central to trial success. Specialized sites will already have tailored SOPs, IRB familiarity, and support teams trained in these lifecycle nuances—ensuring safer and more ethically sound data collection.
Rare Disease Registries and Patient Access
Accessing qualified participants for rare disease trials hinges on registry integration and referral networks, not walk-in recruitment. Top-performing sites maintain their own disease registries or have partnerships with global databases like EURORDIS, NORD, and Global Genes. These registries are pre-vetted for consent, include genotype/phenotype data, and often serve as feeder sources across multiple studies.
In addition, rare disease sites collaborate closely with advocacy groups, local specialists, and international consortia to identify off-label populations or underserved regions with trial-ready participants. This proactive outreach dramatically shortens recruitment cycles while preserving diversity and inclusion benchmarks. Without registry access and stakeholder alignment, many studies fail to enroll even after site activation.
| Capability Area | Biomarker Analysis & Genomic Sequencing | Pediatric & Geriatric Specialization | Registry Integration & Patient Access |
|---|---|---|---|
| Core Role | Enables precise patient stratification and trial eligibility based on genetic markers and biomarkers. | Supports age-specific protocol execution for the two most vulnerable demographics in rare disease trials. | Powers targeted recruitment and improves enrollment speed through access to qualified, pre-consented cohorts. |
| Site Infrastructure Needs | Next-generation sequencing (NGS), qPCR, multi-omics, variant interpretation tools, and validated lab pipelines. | Pediatric-sized equipment, child assent workflows, mobility support, geriatric dosing strategies, and access to psychologists and functional assessment tools. | Internal registries or partnerships with EURORDIS, NORD, Global Genes; real-time referral systems linked to advocacy groups and disease specialists. |
| Sponsor Benefits | Faster enrollment, accurate biomarker targeting, and compliance with genomic research protocols and biobank regulations. | Reduced protocol deviations, improved safety oversight, and higher data quality in complex age-specific populations. | Shortened recruitment timelines, improved inclusion metrics, and consistent access to study-ready participants. |
| Trial Types That Rely on This Capability | Rare oncology, neurometabolic diseases, primary immunodeficiencies, and gene therapy trials. | Lysosomal storage disorders, pediatric neurology, age-related amyloidosis, and multi-generational inherited disease studies. | Studies involving small or ultra-orphan populations, founder mutations, or trials requiring ethnically/genetically diverse cohorts. |
Worldwide Directory of Rare Disease Research Sites
United States (NIH Rare Diseases Network, Mayo Clinic)
The United States remains a global leader in rare disease clinical research due to dedicated networks, high regulatory alignment, and advanced infrastructure. The National Institutes of Health (NIH) spearheads the Rare Diseases Clinical Research Network (RDCRN), which supports more than 20 collaborative consortia across over 100 academic institutions. These sites specialize in disease-specific natural history studies, biomarker validation, and longitudinal follow-up protocols. Each center is embedded within a patient advocacy framework, giving sponsors immediate access to pre-consented, registry-linked cohorts.
The Mayo Clinic, meanwhile, integrates rare disease research into its Center for Individualized Medicine. With in-house genome sequencing labs and trial-design partnerships, it handles both pediatric and adult rare indications with end-to-end precision—from genetic counseling to post-trial patient transitions. Its centralized IRB and adaptive platform protocols make it a high-performing choice for Phase I–III trials.
Europe (UK’s GOSH, Germany’s DRFZ)
Europe’s regulatory landscape and single-payer health systems make it ideal for cross-border rare disease studies. In the UK, Great Ormond Street Hospital (GOSH) leads the field in pediatric rare trials, operating alongside the NIHR Great Ormond Street Biomedical Research Centre. GOSH is known for embedding trials directly into clinical care, especially for rare congenital and metabolic disorders. Its Child Health Research Centre includes high-resolution imaging, pharmacokinetics, and pediatric anesthesiology for trials requiring sedation.
In Germany, the German Rheumatism Research Center (DRFZ) plays a major role in autoimmune and inflammatory rare disease studies. DRFZ combines real-world cohort studies, omics-based research, and biorepository linkage across adult populations. It collaborates with Charité University Hospital and coordinates pan-European consortia through the EU-funded E-Rare program. These sites offer deep academic bench strength, centralized ethics submission, and streamlined pharmacovigilance reporting.
Asia-Pacific (Japan, Singapore, India)
In the Asia-Pacific region, rare disease site performance varies by national infrastructure—but standout hubs are emerging. Japan’s National Center for Neurology and Psychiatry (NCNP) is renowned for its focus on neurogenetic disorders and pharmacogenomic-driven protocols. NCNP integrates its patient database with Japan’s Initiative on Rare and Undiagnosed Diseases (IRUD), allowing sponsors access to well-characterized cohorts.
Singapore’s National University Hospital (NUH) anchors the region’s precision medicine trials. It supports rare oncology, endocrine, and hematological trials with embedded biostatistics and whole-exome sequencing tools. NUH’s co-location with the Genome Institute of Singapore ensures tight coupling between diagnostics and trial eligibility screening.
India’s All India Institute of Medical Sciences (AIIMS) has also risen as a rare disease trial site. It participates in consortia focused on lysosomal storage disorders, congenital syndromes, and rare infectious diseases. Despite regulatory hurdles, AIIMS offers access to genetically diverse populations and low-cost trial operations, making it ideal for early-phase multicenter studies.
Latin America and Middle East
In Latin America, rare disease research sites are emerging through academic-municipal collaborations. Instituto da Criança at the University of São Paulo is notable for its work in pediatric rare oncology and skeletal dysplasia trials. Brazil’s inclusion in the International Rare Diseases Research Consortium (IRDiRC) enables global study alignment and patient data sharing.
In the Middle East, King Faisal Specialist Hospital & Research Centre (Saudi Arabia) and Sheba Medical Center (Israel) lead in rare disease research. King Faisal operates a genomics lab specializing in founder mutations prevalent in consanguineous populations. Sheba’s Institute for Rare Diseases supports ultra-orphan studies, with AI-driven patient identification across its national health system. Both institutions offer registry-connected cohorts, biobanking capabilities, and high trial compliance, positioning them as strategic hubs for regionally tailored protocols.
| Region | Top Sites & Infrastructure Highlights |
|---|---|
| United States | NIH RDCRN supports over 20 rare disease consortia across 100+ academic institutions, embedding trials within advocacy-driven, registry-linked ecosystems. Mayo Clinic offers integrated genomics, centralized IRB, and full lifecycle coordination for adult and pediatric trials under its Center for Individualized Medicine. |
| Europe | UK’s GOSH leads pediatric trials via the NIHR Biomedical Research Centre, embedding research in care pathways for congenital and metabolic conditions. Germany’s DRFZ collaborates with Charité and pan-European consortia to deliver autoimmune and inflammatory disease trials with strong real-world data integration. |
| Asia-Pacific | Japan’s NCNP specializes in neurogenetics, linking with the IRUD registry for precise patient targeting. Singapore’s NUH, co-located with the Genome Institute, supports rare oncology and endocrine trials through exome sequencing and trial-ready diagnostics. India’s AIIMS provides access to genetically diverse populations and runs early-phase trials on lysosomal and infectious diseases. |
| Latin America | Instituto da Criança (Brazil) focuses on pediatric rare oncology and skeletal conditions; Brazil’s membership in IRDiRC boosts global collaboration and data exchange. |
| Middle East | King Faisal Hospital (Saudi Arabia) targets founder mutations through advanced genomic profiling. Sheba Medical Center (Israel) supports ultra-orphan trials using AI-led national patient identification and registry-linked cohorts. |
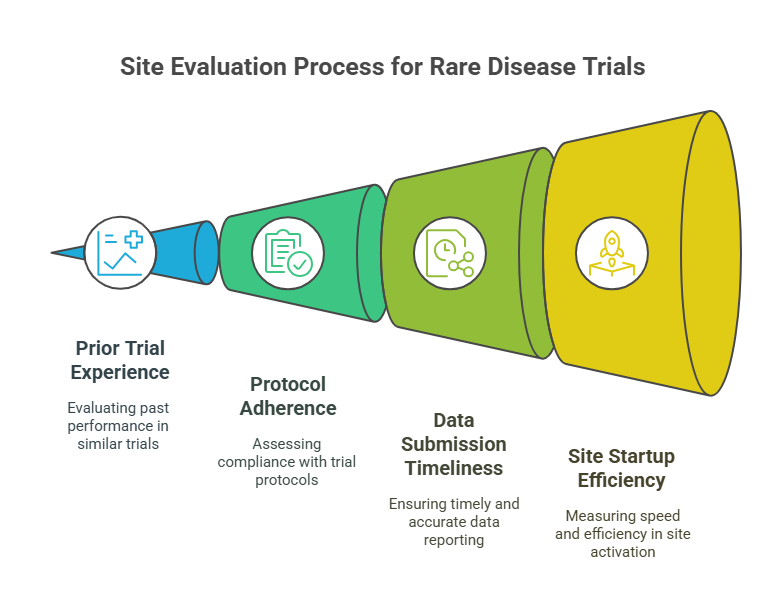
Key Metrics Sponsors Use to Evaluate These Sites
Prior Trial Experience and Enrollment Rates
When selecting rare disease research sites, sponsors prioritize historical performance—particularly in low-N trials requiring genetic screening and longitudinal follow-up. Sites with documented success in orphan-designated studies demonstrate not only operational capability but also an understanding of rare protocol design, subject retention, and ethical compliance.
Enrollment efficiency is especially critical. A site may only need to recruit 5–10 patients, but delays of even two weeks per subject can cripple timelines. Sponsors assess average time from site activation to first patient-in (FPI) and compare that against industry benchmarks. Sites that already maintain disease-specific registries or advocacy partnerships tend to outperform others on both speed and quality of enrollment. Prior success in rare trials with FDA Fast Track or EMA PRIME designations is often seen as a strong predictive indicator.
Protocol Adherence and Data Submission Timelines
Rare disease trials involve multi-arm protocols, off-label drug administration, and biomarker-based endpoints, requiring meticulous adherence. Sponsors evaluate a site's deviation history and audit reports to determine consistency. Even minor protocol violations in rare trials can invalidate cohorts, so performance in previous high-complexity studies is weighted heavily.
Data timeliness also plays a key role. Delays in EDC entry, lab uploads, or SAE reporting can jeopardize sponsor timelines or regulatory submissions. High-performing sites will have dedicated data managers, automated QC systems, and centralized reporting structures. Those with experience in Phase I/II crossover trials or expanded access programs tend to excel in maintaining real-time data pipelines.
Site Startup and Ethics Approval Timelines
Startup timelines are a bottleneck in rare disease research. Sponsors assess how quickly a site can complete feasibility surveys, budget negotiations, and ethics approval. Sites with central IRB alignment or in-house ethics boards can often activate within 30–45 days—far faster than those navigating multi-layer institutional reviews.
Additionally, sponsors measure the average duration between contract signature and regulatory greenlight, a KPI that reflects internal coordination strength. Sites that offer pre-negotiated templates, rapid budget iterations, and dedicated regulatory staff gain favor. Many top-performing sites in rare trials also offer early parallel startup, where training and document prep begin before full ethics clearance to compress timelines.
Technology and Data Infrastructure at Rare Disease Sites
EDC Systems, Remote Monitoring, and Imaging Capabilities
Rare disease sites require robust digital ecosystems to manage complex data flows, small sample sizes, and real-time compliance tracking. At a baseline, sites must use validated Electronic Data Capture (EDC) systems with built-in audit trails, automated alerts, and modular CRF design—especially critical when protocols evolve mid-study. Sponsors often look for platforms like Medidata Rave or Veeva Vault, which support custom endpoints and genomic annotation modules.
Remote monitoring is also non-negotiable. Given the global dispersion of patients in rare disease trials, sponsors require sites to offer secure VPN-based CRA access, remote SDV workflows, and source upload integrations. High-performing sites even deploy eSource and eConsent tools, ensuring that compliance and data fidelity are preserved even in decentralized settings.
Advanced imaging is another differentiator. Rare neuromuscular, skeletal, or metabolic trials often depend on longitudinal MRI, PET-CT, or DXA scans as efficacy markers. Sites with imaging core lab partnerships, DICOM integration, and automated image QC pipelines accelerate endpoint analysis while maintaining regulatory-grade data quality.
Integration with Global Rare Disease Databases
What sets elite rare disease research sites apart is their interoperability with global data networks. Sites linked to databases like Orphanet, the RD-Connect Genome-Phenome Platform, and NORD IAMRARE can pre-screen patients more efficiently and contribute to broader post-trial analytics. These integrations also enable automated eligibility flagging, historical control comparisons, and real-world evidence generation.
Sites must also maintain FHIR-compliant data structures that support rapid export, de-identification, and API-based submission to registries or sponsors. This is especially important in studies using master protocols, where new sub-studies launch mid-trial and require dynamic subject reclassification.
Ultimately, the best-performing sites treat data architecture not as back-office infrastructure but as a strategic asset, actively shaping how quickly, securely, and transparently rare disease trials progress.
| EDC Systems, Remote Monitoring & Imaging | Global Database Integration & Interoperability |
|---|---|
| Digital Platforms Sites use validated EDC systems like Medidata Rave and Veeva Vault, designed for biomarker-driven trials and mid-study protocol changes. |
Global Data Linkage Top sites integrate with Orphanet, RD-Connect, and NORD IAMRARE for automated patient matching and cross-trial insights. |
| Remote Capabilities CRAs require secure VPN-based access, remote SDV workflows, eSource, and eConsent to support decentralized trial designs. |
FHIR Compliance Data systems must support FHIR architecture with export-ready formats, de-identification pipelines, and registry APIs. |
| Imaging Infrastructure Rare neuromuscular or metabolic trials rely on MRI, PET-CT, and DXA scans. Sites must offer DICOM integration and automated quality control. |
Adaptive Protocol Readiness Infrastructure enables mid-trial sub-study launches, eligibility reclassification, and real-time analytics to support master protocols. |
| Strategic Value Sites with this stack ensure data accuracy, regulatory compliance, and real-time visibility—especially critical in global, long-duration studies. |
Strategic Value Data isn’t siloed—it drives faster enrollment, broader impact analysis, and accelerates regulatory submission across rare disease portfolios. |
How the Certified Clinical Research Coordinator (ACRCC) Course Helps You Land a Role in Rare Disease Research
Site Roles Like CRCs, Data Managers, Trial Liaisons
Rare disease research sites rely heavily on multi-functional clinical staff who can adapt to high-complexity, low-volume studies with rigorous compliance needs. Clinical Research Coordinators (CRCs) are central to this model. In rare disease trials, a CRC is expected to manage patient registries, coordinate genetic screening workflows, track off-site procedures, and serve as the daily communication bridge between patients and sponsors.
Data Managers play an equally vital role. With trials depending on rare variant confirmation, biomarker trends, and small-sample data integrity, sites need data professionals who can handle both the technical platforms and regulatory nuances of orphan trials. Trial Liaisons, often acting as advocacy or family coordinators, are also crucial in studies requiring high-touch engagement and decentralized visit planning.
These are not entry-level jobs with generic SOPs. They demand professionals trained to navigate ethics-heavy, protocol-adaptive environments—a skillset few gain without focused education like the Certified Clinical Research Coordinator (ACRCC) course.
What You Learn in ACRCC That Applies Here
The ACRCC program, offered by the Certified Clinical Research Professionals Society (CCRPS), is tailored for high-impact roles in rare and complex trials. It includes training in genetics-informed consent, decentralized trial coordination, and pharmacovigilance workflows for orphan drugs. These modules are critical when working at specialized sites where trials involve pediatric assent, remote data capture, and regulatory submissions for early-phase indications.
The course also equips learners with site-readiness tools: budget negotiation tactics, site qualification visit preparation, risk-based monitoring strategies, and audit defense planning. These are the day-to-day demands placed on CRCs working in elite rare disease environments.
ACRCC also emphasizes the ethical dimensions unique to these trials—like managing family-reported outcomes, crossover trial complexities, or progressive disease monitoring. Graduates enter the workforce not just job-ready, but rare-disease ready.
Frequently Asked Questions
-
To work at a rare disease site, you typically need a background in life sciences, nursing, pharmacy, or clinical research—but specialization is key. Employers prefer candidates who have completed clinical research certifications like the ACRCC from CCRPS. Since rare trials involve complex protocols and vulnerable populations, recruiters look for experience in informed consent, patient interaction, and EDC platforms. If you're aiming for a CRC role, additional knowledge of genomics, orphan drug development, or pediatric trial design can significantly boost your eligibility. Prior work with IRBs or registry-based trials is also a strong asset.
-
Rare disease sites are built around specialization. They often serve smaller, genetically defined populations and require advanced diagnostics, such as sequencing or biomarker profiling. Unlike general trial sites that may run dozens of large-scale studies at once, rare disease sites prioritize deep phenotyping, longitudinal follow-up, and patient-centric care models. Their staff is trained to handle ethical complexities like pediatric assent, and these sites frequently collaborate with global registries, advocacy organizations, and centralized IRBs. Technology infrastructure also tends to be more advanced, especially for remote trials and imaging.
-
The most active countries include the United States, United Kingdom, Germany, Japan, Singapore, and Brazil. The U.S. leads through its NIH Rare Diseases Network, while the UK excels in pediatric rare trials via Great Ormond Street Hospital. Germany's DRFZ focuses on autoimmune conditions, and Japan leverages national genomics programs. Singapore provides a tech-forward ecosystem for precision trials, and Brazil stands out for pediatric and underserved population studies. These countries have dedicated rare disease centers, regulatory frameworks, and data-sharing capabilities, making them hubs for sponsor engagement.
-
Recruitment at rare disease sites relies heavily on patient registries, genomic databases, and advocacy partnerships. Unlike general trials that use advertisements or hospital referrals, rare disease sites tap into pre-consented cohorts from organizations like NORD, Global Genes, and national genomic initiatives. They also use AI-driven screening, collaborate with disease-specific clinicians, and host education campaigns targeting rare communities. Many sites maintain internal registries that include genotype and phenotype data, enabling precise pre-screening. Recruitment is proactive, multi-institutional, and built on trust with patient families and support networks.
-
Rare disease trials use Electronic Data Capture (EDC) systems, remote monitoring tools, genomic sequencing platforms, and advanced imaging systems. EDC tools like Medidata Rave or Veeva Vault help maintain clean data with audit trails and customized CRFs. Sites also use eSource, eConsent, and decentralized trial platforms for remote patient interaction. Imaging capabilities are often critical—especially for neuromuscular or skeletal diseases—so DICOM integration and QC pipelines are standard. Some sites also use API-based integration with rare disease databases for eligibility and data sharing.
-
Academic and national centers offer the infrastructure, ethics familiarity, and multidisciplinary teams needed for rare disease research. These trials often involve first-in-human dosing, complex endpoints, and multi-year follow-up, which community hospitals typically aren’t equipped to handle. Academic sites often have in-house sequencing labs, centralized IRBs, and translational research units. They’re also involved in consortia that fund or co-develop protocols, giving them early access to trial opportunities and registry partnerships. Sponsors favor these settings for their regulatory readiness and scientific depth.
-
Yes—but you'll need targeted training. While direct experience helps, completing a focused program like the Certified Clinical Research Coordinator (ACRCC) shows that you understand genetic consent, registry coordination, and orphan drug protocols. Many sites offer on-the-job training, but they favor applicants who show preparedness for decentralized trials, vulnerable populations, and data integrity workflows. Volunteering with advocacy groups, working in pediatric or neurology clinics, or handling ethics documents at general trial sites are also great stepping stones into this field.
-
Rare disease trials tend to be longer and more complex than general trials. While a typical Phase II oncology trial might run for 12–18 months, rare disease trials often extend over 3 to 5 years, especially those involving natural history cohorts or pediatric development plans. This is because patient populations are limited, and long-term safety and efficacy data are essential for regulatory approval. In many cases, trials transition into expanded access programs or rolling submission studies, which further extend site responsibilities.
Final Thoughts
Rare disease trials aren’t just smaller—they’re fundamentally different. Success depends on research sites with specialized capabilities, rare-patient access, and genomic infrastructure built into their daily operations. From startup speed to biomarker handling, these sites operate on a level that general institutions rarely match. Their strength lies not in volume, but in precision, ethical sensitivity, and long-term patient relationships.
For professionals aiming to contribute meaningfully to this field, generic training won’t suffice. Certification programs like the Certified Clinical Research Coordinator (ACRCC) equip you with the regulatory insight, trial coordination skills, and rare disease-specific knowledge sites now demand. Whether you’re building a career or designing a global trial, your choice of site—or your readiness to join one—can directly impact the future of therapeutic discovery for rare diseases.