Clinical Trial Budget Oversight: Project Manager’s Best Practices
Budgeting in clinical trials is more than tracking expenses—it’s about controlling risk, timelines, and expectations. Project managers (PMs) must align financial oversight with trial operations, regulatory requirements, and sponsor goals.
Even a single protocol amendment or recruitment delay can send costs soaring. Without proactive budget planning, unexpected issues like adverse events or slow site activation can derail timelines and inflate budgets.
As trials become more global and technology-driven, PMs must manage costs across multiple platforms—EDC, CTMS, and remote teams. This demands real-time visibility and adaptability.
This guide offers practical tools and techniques for PMs to build smarter budgets, respond to risk, and report with clarity—ensuring trials stay on track without compromising quality.
Why Budget Oversight Matters in Clinical Trials
Cost Drivers That Can Derail a Trial Budget
Some of the most damaging cost overruns stem from events that aren't budgeted accurately. Protocol amendments, unexpected adverse events (AEs), or site-level deviations can rapidly consume contingency funds. For example, adverse events not only affect patient safety but often require additional tests, unplanned procedures, or medical follow-ups—all unbudgeted if risk planning wasn’t thorough.
Other hidden cost drivers include delayed IRB approvals, underperforming sites, and tech implementation failures. These delays don’t just push timelines—they trigger renegotiations, staffing adjustments, and emergency vendor fees. PMs must learn to expect variability and account for it in early budget drafts.
Strategic Importance for Sponsors and CROs
For CROs and sponsors, budget oversight directly impacts business continuity and portfolio ROI. Sponsors expect predictability and clear justifications for every variance. CROs, on the other hand, must deliver services profitably while staying within budget caps.
Poor budget communication between PMs, CROs, and sponsors often leads to trust issues or project reassignment. Effective PMs use budget data to drive transparency, flag risks early, and justify adaptive planning—turning financial fluency into a leadership asset.
Budget Oversight: The PM’s Financial Shield
Hidden Costs: Unexpected AEs, site deviations, and delayed IRB approvals can trigger serious overruns—fast.
Sponsor Expectations: Predictability, accountability, and transparency are non-negotiable in financial reporting.
PM Advantage: Budget-smart project managers earn trust by using data to justify pivots, control risk, and protect ROI.
Tip: Track budget-impacting events in real time and communicate variance early. Silence erodes sponsor trust faster than a missed deadline.
Building a Budget Foundation from Trial Protocol
Aligning Costs with Study Design
The clinical trial protocol isn’t just a blueprint for operations—it’s the foundation of the entire budget. Every inclusion criterion, lab test, site visit, and dosing schedule carries a cost. A complex protocol means more investigator time, additional patient monitoring, and expensive tech integrations. That’s why aligning costs with protocol design is one of the most crucial steps for a PM.
For example, a protocol requiring imaging at multiple timepoints across multiple sites will demand higher vendor and logistics fees. PMs must translate protocol expectations into line-item realities, engaging finance teams and vendors early to avoid underbudgeting.
Accounting for Regulatory Milestones
Many teams underestimate the costs associated with Institutional Review Board (IRB) processes and regulatory milestones. From initial submission fees to amendments and renewals, IRB timelines directly impact trial initiation and site activation spend. PMs need to plan for delays and buffer for potential resubmissions.
Understanding IRB responsibilities helps PMs map regulatory checkpoints onto payment schedules, keeping budgets aligned with compliance demands. Early missteps in IRB budgeting can ripple into delayed site launches, wasted staffing, and out-of-pocket overheads.
| Budget Area | Key Considerations | Impact on Trial Budget |
|---|---|---|
| Protocol Design | Includes criteria, tests, site visits, procedures, and technology requirements | More complexity = higher costs for monitoring, investigator time, and vendors |
| Imaging & Diagnostics | Multiple imaging timepoints across sites | Increases vendor, coordination, and logistics fees |
| IRB Processes | Submission, approval, amendments, renewals | Delays and resubmissions can inflate start-up costs |
| Regulatory Milestones | Mapped against payment schedules and site activation | Poor planning leads to staffing gaps and out-of-pocket overhead |
Cost Monitoring Across Trial Phases
Budget Planning for Phase I–IV Variability
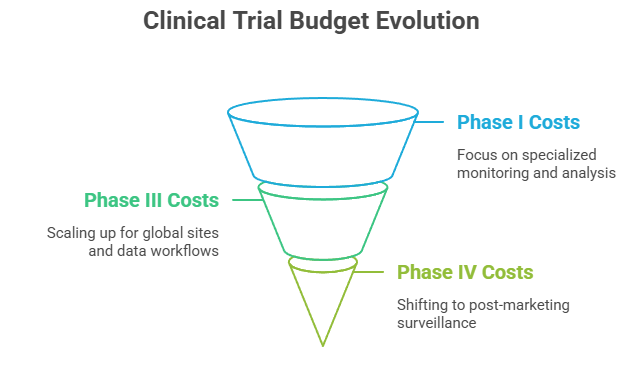
Each clinical trial phase introduces different financial demands. Phase I trials often require specialized monitoring environments, custom lab analysis, and 24/7 clinical oversight—making them costly per patient. As trials scale into Phase III, the budget must account for dozens of global sites, complex data workflows, and extended monitoring windows.
By Phase IV, spending shifts toward post-marketing surveillance, safety reporting systems, and broader regional tracking. Project managers must budget for this evolution, adjusting for varying tech costs, monitoring intensity, and regulatory reporting.
Reviewing real-world guides like the Phase I Clinical Trials: Objectives, Risks & Process, the Phase III Clinical Trials – Definitive Guide, and the Phase IV Clinical Trials – Post-Marketing Studies can help PMs anticipate cost spikes across phases.
Key Differences in Budget Line Items Per Phase
Trial phases also differ in how the money is spent. Early-stage trials budget heavily for inpatient safety procedures and dose escalation analytics. Mid-stage trials (Phase II/III) reallocate costs toward broader site monitoring, EDC licensing, and statistical services. In Phase IV, expenses tilt toward long-term follow-ups, signal detection software, and adverse event tracking platforms.
The most effective PMs anticipate these shifts and adjust budget allocations proactively, avoiding delays caused by misaligned financial planning.
Budgeting Tools and Tracking Systems for PMs
CTMS and EDC Integration
Effective budget oversight depends on real-time visibility across operations. This is where Clinical Trial Management Systems (CTMS) and Electronic Data Capture (EDC) tools play a central role. By integrating budgeting workflows with site activity, protocol milestones, and data entry status, PMs can flag overspending early—before it turns into a full-blown issue.
Modern CTMS platforms allow teams to track financial KPIs alongside operational metrics, making it easier to sync milestone-based payments and vendor costs. For a comparative look at options, see the Top 20 Clinical Trial Management Systems (CTMS): Reviewed and Compared 2025.
Meanwhile, EDC platforms feed real-time visit data and patient enrollment rates directly into budget dashboards—allowing faster and more accurate forecasting.
CDMS and Real-Time Budget Adjustments
Clinical Data Management Systems (CDMS) also play a budgetary role, particularly in managing the cost of query resolution, data cleaning, and third-party vendor integration. With today’s advanced platforms, PMs can automate reconciliation processes and instantly visualize burn rate fluctuations.
The Clinical Data Management Systems (CDMS) Directory helps teams choose tools that support both regulatory compliance and financial control—crucial for staying within budget during intensive database lock or audit prep phases.
Risk Management in Budget Oversight
Preemptive Budget Risk Mitigation Plans
Project managers who plan for risks before they materialize are more likely to deliver trials on budget. This starts with developing risk-adjusted budget buffers—allocations for unexpected recruitment delays, vendor changes, or regulatory pushbacks.
Risk mitigation isn’t just theoretical. PMs can consult real-world frameworks like the Risk Management Plans (RMPs): Comprehensive Pharmacovigilance Guide to proactively design contingency strategies. These include tiered funding models, milestone-linked payments, and change order response protocols.
Early stakeholder alignment on these contingencies often determines whether a trial stays afloat or sinks financially.
When Forecasts Fail – Recovery Tactics for PMs
Even with the best planning, some trials overshoot projections. When that happens, PMs need recovery tactics. These can include burn rate monitoring, emergency site funding reallocations, or renegotiating deliverables with sponsors.
Having a structured fallback plan in place allows PMs to maintain sponsor trust and regain control. Strategies from the Risk Management in Clinical Trials: PM’s Comprehensive Guide offer practical examples of how to assess deviations and correct course without derailing the full budget.
Budget Risk Oversight: Critical Focus Areas
Preemptive Mitigation Plans
Allocate buffers for unexpected events like recruitment delays or vendor switches. Build tiered funding structures and milestone-triggered payments to stay flexible.
Use resources like the Pharmacovigilance RMP guide to structure compliant contingency models.
Recovery Tactics When Budgets Slip
When overspending hits, rely on burn-rate monitoring, emergency site fund shifts, and renegotiation of timelines or deliverables.
Learn how to reset effectively with strategies from the Risk Management in Trials Guide.
Best Practices for Budget Reporting and Stakeholder Communication
Aligning Financial Reports with Sponsor Expectations
A clinical trial budget isn’t just an internal document—it’s a trust-building tool between the project manager and the sponsor. Sponsors expect clear, accurate, and timely reporting that matches contractual terms and milestones. That includes forecasting variances, explaining unexpected costs, and linking expenditures to trial outcomes.
To meet these expectations, PMs must understand sponsor priorities—whether it's recruitment efficiency, rapid site activation, or regulatory alignment. Resources like the Best Clinical Trial Sponsors in the US: Comprehensive Directory and Insights help PMs tailor financial communication to sponsor type and operating model.
A polished financial summary builds sponsor confidence and improves the likelihood of long-term collaboration.
Tools for Transparent Budget Dashboards
Modern budget dashboards go beyond Excel. PMs now use real-time, visual budget tools to present financial data in a format that’s easily digestible by stakeholders. These dashboards—often integrated into CTMS platforms—display cost burn, milestone payments, and budget vs. actuals in one place.
Dashboards also reduce communication errors. When sponsors and CROs view the same data in real time, there’s less back-and-forth and fewer misunderstandings. Linking dashboards to portals allows stakeholders to self-serve updates, saving PMs hours of status reporting each month.
Final Thoughts
Clinical trial budget oversight is more than spreadsheets and cost checks—it’s a strategic function that can make or break trial success. Project managers who understand this don’t just manage money—they steer the entire study toward timeline stability, regulatory alignment, and stakeholder satisfaction.
From aligning protocol design with budget forecasts to adjusting for phase-specific variability, the most effective PMs apply clinical insight, financial strategy, and digital tools in equal measure. Real-time dashboards, clear reporting, and proactive risk plans are no longer optional—they’re the baseline for sponsor trust.
When budgets are monitored with precision, trials avoid costly delays, recruitment gaps, and compliance risks. The result? Higher-quality data, faster submissions, and stronger sponsor relationships.
Ultimately, budget mastery is project mastery. PMs who lead with financial fluency and adaptability position their trials—and careers—for long-term impact.
Frequently Asked Questions
-
The first step is aligning the clinical trial protocol with cost projections. Every procedure, visit, and data point in the protocol has a financial implication. PMs should collaborate early with finance teams and operational leads to ensure that complex trial designs don’t exceed feasible funding. Tools like protocol-specific cost templates and feasibility scorecards help ground this process in realistic planning.
-
When costs rise unexpectedly—due to delays, site changes, or adverse events—PMs must log deviations, notify sponsors, and provide clear justifications. Real-time budget tracking through CTMS or CDMS platforms helps PMs spot cost variances early. Compliance is maintained by ensuring audit trails, updated vendor contracts, and change control documentation align with Good Clinical Practice (GCP) standards.
-
Some frequently missed budget items include site startup delays, screen failure reimbursements, courier fees for lab samples, and patient retention strategies. Additionally, global trials often incur hidden translation, customs, and import/export costs. Including buffer amounts for each major category helps manage unexpected spikes without halting operations.
-
Most sponsors expect monthly budget updates with clear variance explanations and milestone-based burn rate visuals. In high-risk studies, biweekly check-ins may be needed. Dashboards linked to CTMS platforms can auto-generate sponsor-facing summaries, while PMs supplement this with narrative reports on spend risk and corrective actions.
-
CTMS dashboards are the most common, but advanced PMs also use budget-specific visualizations like heat maps, phase-by-phase cost graphs, and predictive analytics from integrated CDMS systems. These tools help link financial data to operational progress, making budget conversations easier with non-financial stakeholders.