GCP Guidelines Mastery: Essential Practices for Clinical Trials
Good Clinical Practice (GCP) isn’t just a regulatory checkbox — it’s the backbone of credible, ethical clinical trials across the globe. Whether you're designing a protocol, collecting source data, or reviewing adverse events, mastery of GCP standards ensures participant safety, data validity, and regulatory approval. In a landscape where a single deviation can result in trial rejection or legal penalties, understanding GCP isn't optional — it's essential.
The ICH-GCP framework, adopted by the FDA, EMA, and other global regulatory bodies, lays out specific guidelines for investigators, sponsors, and monitors. Mastery of these guidelines isn't just for compliance — it's a competitive edge. Those certified in GCP are trusted with leadership roles in clinical research, site management, and regulatory affairs. The right training sharpens your ability to navigate complex audits, manage ethical responsibilities, and align documentation with international standards. For professionals ready to lead with confidence, the Good Clinical Practice Certification by CCRPS offers a deep, practical understanding of what it takes to get trials approved — and keep them inspection-ready.
What Are GCP Guidelines and Why Do They Matter?
Good Clinical Practice (GCP) refers to the internationally accepted ethical and scientific quality standards for designing, conducting, recording, and reporting clinical trials involving human participants. These guidelines are enforced by regulatory authorities like the FDA, EMA, and MHRA, and form the baseline for ensuring trial integrity, subject safety, and data reliability.
At its core, GCP protects the rights, safety, and well-being of trial subjects. Every element of a clinical trial — from protocol development to final data analysis — must be handled in a way that respects participant autonomy and meets regulatory demands. These aren’t just theoretical ideals. GCP principles are legally binding, and noncompliance can lead to disqualification, rejected submissions, or legal action.
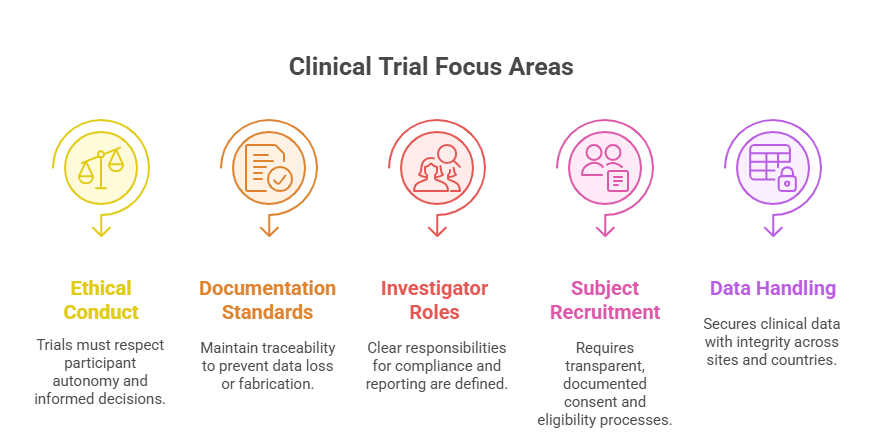
Key Areas Covered by GCP
Ethical Conduct: Ensures clinical trials are run with full respect for individuals, cultural differences, and informed autonomy.
Documentation Standards: Enforces traceability of every action, decision, and data point to prevent fabrication or loss.
Accountability for Investigators and Sponsors: Outlines roles in maintaining compliance, submitting safety reports, and ensuring trial continuity.
Subject Recruitment & Consent: Requires clear processes for consent, eligibility, and documentation, all traceable and reviewable.
Data Handling & Confidentiality: Stipulates secure, accurate, and confidential handling of clinical data — including during multi-center and multinational trials.
Why GCP Still Matters in 2025 and Beyond
Despite advances in AI, eSource platforms, and decentralized trials, GCP compliance remains non-negotiable. New technologies have introduced efficiencies, but also vulnerabilities. Regulators expect digital compliance to uphold traditional GCP expectations. Failing to account for electronic audit trails or participant e-consent mechanisms can still lead to serious violations.
In an era of globalized trials and increased regulatory scrutiny, GCP ensures universal standards are met regardless of geography or sponsor type. It also acts as a common language — allowing CROs, sponsors, and research sites to collaborate across regulatory jurisdictions.
Mastering GCP guidelines means mastering trial legitimacy. Whether you're a monitor, coordinator, or principal investigator, fluency in GCP is the most critical prerequisite for career growth and trial success.
Informed Consent
Informed consent is not a one-time form — it’s a continuous, documented conversation between the investigator and the participant. Under ICH-GCP, a subject must be fully informed about the study’s nature, risks, benefits, and their right to withdraw at any time without penalty.
The consent form must be written in language understandable to the participant and approved by an ethics committee. It must explain the experimental nature of the study, alternative treatments, confidentiality measures, and compensation protocols.
Verbal explanations are required alongside the written form, and the process must be documented with signatures and dated copies retained. For minors or incapacitated individuals, legal representatives must be involved. If new information arises mid-trial, re-consent is mandatory.
Failure to follow proper informed consent procedures is considered a serious GCP violation — and often leads to regulatory rejection of the data collected from that subject.
Core Principles of ICH-GCP Compliance
The International Council for Harmonisation’s Good Clinical Practice (ICH-GCP) guidelines form the universal gold standard for clinical trials. Adopted by regulatory agencies like the FDA, EMA, PMDA, and Health Canada, these principles ensure that trials are ethical, scientifically sound, and generate verifiable data. For professionals navigating cross-border studies, ICH-GCP compliance is non-negotiable for global acceptance.
1. Protection of Human Subjects
Every GCP-compliant trial must uphold the dignity, rights, safety, and well-being of participants. This is not symbolic — it is backed by rigorous protocol requirements, mandatory informed consent, and safety monitoring. Trials must demonstrate that the potential benefits outweigh risks, and risks must be minimized through careful design and oversight.
Ethics committees (IRBs or IECs) play a central role, reviewing study protocols, participant materials, and amendments. No trial involving human subjects should begin without documented ethical approval.
2. Scientifically Valid Protocols
GCP requires that studies be based on sound scientific principles. This includes:
Clearly defined objectives and endpoints
Statistically valid sample sizes
Risk mitigation strategies
Transparent methodology
Deviation from protocol must be documented, justified, and, when necessary, reported to regulators. A scientifically unsound trial, even with ethical clearance, is non-compliant with GCP.
3. Roles and Responsibilities Are Clearly Defined
The guidelines strictly delineate what sponsors, investigators, monitors, and IRBs are each responsible for. This prevents overlap, gaps, and liability issues. For instance:
Sponsors must ensure trial funding, supply chain, monitoring, and data analysis systems are in place.
Investigators are responsible for on-site conduct, informed consent, and source documentation.
Monitors ensure real-time oversight and audit-readiness.
No aspect of trial management should operate in a gray zone. Defined accountability is a core pillar of audit readiness.
4. Data Accuracy and Recordkeeping
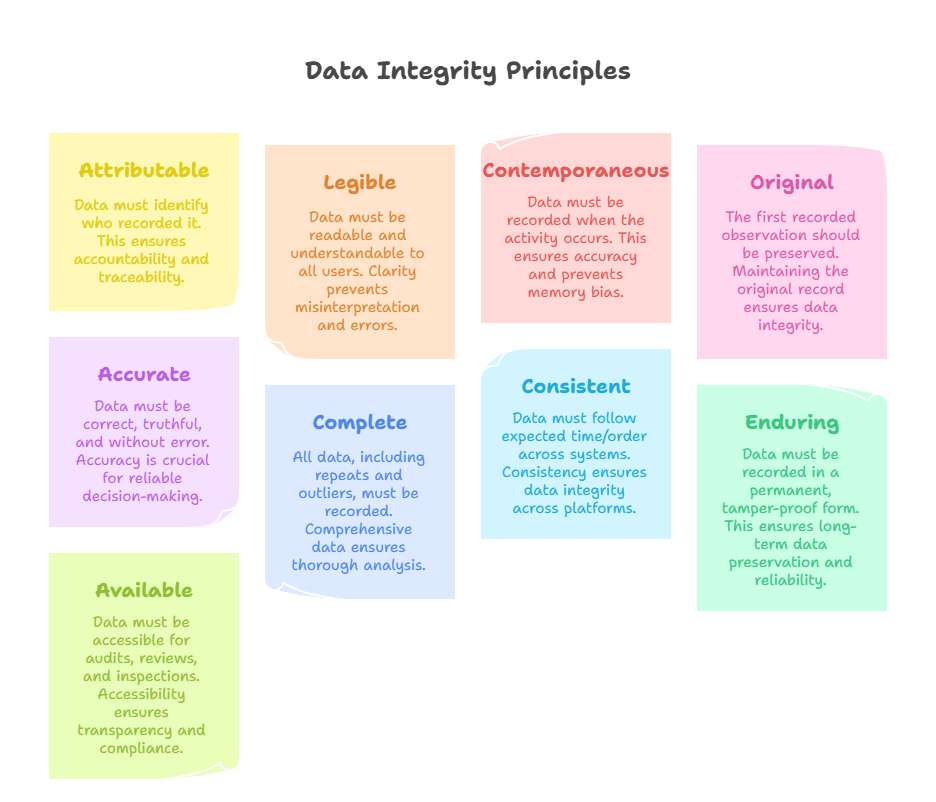
GCP emphasizes complete, accurate, and timely documentation. This includes the ALCOA+ principles:
Attributable
Legible
Contemporaneous
Original
Accurate
+Complete, Consistent, Enduring, and Available
These records must be preserved in a format that enables inspection and verification by regulatory authorities, even years after trial closeout. Failure to maintain audit trails is one of the most common inspection findings globally.
5. Quality Systems and Risk-Based Monitoring
Modern GCP standards require sponsors and CROs to implement Quality Management Systems (QMS) that prevent rather than detect issues. This includes risk-based monitoring (RBM), centralized oversight tools, SOPs, deviation logs, and CAPA systems.
Compliance is no longer about manual checks — it’s about predictive controls, preemptive error correction, and integrated oversight systems that prove real-time adherence to GCP.
6. Investigational Product Management
The guidelines also include protocols for investigational drug/device handling:
Proper storage, labeling, accountability, and dispensing
Temperature monitoring and documentation
Return or destruction post-study
Reconciliation of product inventory vs subject use
Any failure in IP tracking can invalidate trial results and prompt regulatory action.
| GCP Principle | Key Requirements | Compliance Highlights |
|---|---|---|
| Protection of Human Subjects |
Ethical review by IRBs/IECs Informed consent process Risk-benefit justification |
No trial may begin without documented ethical approval. Participant safety takes priority over science and commercial interests. |
| Scientifically Valid Protocols |
Clear objectives and endpoints Statistically valid design Risk mitigation plans |
All deviations must be documented and justified. Scientific integrity is foundational to GCP compliance. |
| Defined Roles and Responsibilities |
Sponsor: oversight, funding, systems Investigator: site conduct, documentation Monitor: real-time compliance |
Clear accountability prevents gaps and ensures audit-readiness. No role should operate in ambiguity. |
| Data Accuracy and Recordkeeping |
ALCOA+ principles: Attributable, Legible, Contemporaneous, Original, Accurate +Complete, Consistent, Enduring, Available |
All data must be inspection-ready and retained long-term. Poor documentation is a common FDA/EMA inspection failure. |
| Quality Systems and Risk-Based Monitoring |
QMS implementation Risk-Based Monitoring (RBM) SOPs, CAPA logs, deviation tracking |
Compliance is proactive, not reactive. Predictive controls and centralized oversight are now expected. |
| Investigational Product Management |
Storage, labeling, dispensing Temperature tracking Reconciliation and disposal |
Any lapses in IP tracking can result in trial invalidation or regulatory enforcement. |
Data Integrity and Audit Trails
Data integrity isn’t just a quality benchmark — it’s a regulatory demand that underpins every clinical trial submission. Regulatory agencies like the FDA, EMA, and MHRA base their entire review process on the assumption that your data is accurate, unaltered, and fully traceable. Without verified integrity, even the most effective intervention can be rejected due to uncertainty around its supporting evidence.
ALCOA+ Principles in Practice
ICH-GCP requires that clinical data follow the ALCOA+ framework:
Attributable: Each data entry must clearly show who recorded it.
Legible: Records must be readable for audits, even years later.
Contemporaneous: Data must be entered at the time of the activity.
Original: Source documents must be preserved in their raw form.
Accurate: Entries must reflect true observations without bias.
Complete, Consistent, Enduring, and Available: These final four principles ensure that data isn’t cherry-picked, modified later, or inaccessible when reviewed.
Adherence to ALCOA+ is essential for 21 CFR Part 11 compliance and global regulatory acceptance.
Why Audit Trails Are Non-Negotiable
Every change made to data — whether in eCRFs, lab systems, or investigator notes — must generate an audit trail. That means recording who made the change, when it was made, what was changed, and why. This applies to both electronic and paper systems, though regulators are now especially focused on digital audit capabilities.
Without a functioning audit trail, regulators may:
Invalidate your data
Delay or deny trial approval
Issue 483s or warning letters
Flag the site for re-inspection
Audit trails are not just logs — they are evidence of credibility. Real-time traceability, user access controls, and timestamp validation are now expected by default in electronic systems.
Consequences of Non-Compliance
Trials lacking validated audit systems risk having entire datasets excluded from final submissions. Sponsors may be forced to repeat data collection, and site reputations may be damaged permanently. Regulatory trust is earned through transparency — and audit trails are the proof.
Sponsor and Investigator Responsibilities
Sponsors and investigators carry the legal and operational backbone of any GCP-compliant trial. Their responsibilities are not overlapping — they are complementary and explicitly divided under ICH-GCP to ensure full accountability at every stage of the study.
Sponsor Duties
Sponsors are responsible for designing the trial, selecting qualified investigators, and ensuring regulatory approvals are in place. They must also:
Develop the protocol, case report forms (CRFs), and informed consent templates
Provide investigational product (IP) oversight, including labeling, shipping, and reconciliation
Appoint monitors and auditors to ensure continuous quality control
Report serious adverse events and ensure regulatory submissions are accurate and timely
Failure to do so risks noncompliance and data rejection at the approval stage.
Investigator Duties
Principal Investigators (PIs) are responsible for conducting the trial at their site. Their responsibilities include:
Ensuring ethical conduct, informed consent, and subject safety
Maintaining source documents and regulatory binders
Managing the study team and training sub-investigators
Reporting deviations and adverse events to sponsors and IRBs
PIs are legally accountable for trial conduct at their site, and any oversight failure — even by staff — can trigger inspection findings.
Proper alignment between sponsor and investigator responsibilities is not just about efficiency — it’s central to GCP compliance.
Common Pitfalls in GCP Implementation
Even experienced clinical research teams often misinterpret or underestimate key GCP requirements. These oversights can compromise data validity, trigger inspection findings, or lead to regulatory hold letters. Below are the most frequent — and costly — pitfalls.
Inadequate Informed Consent Documentation
Many sites treat informed consent as a one-time task. But regulators expect ongoing consent communication, especially when protocol changes or new risk information emerges. Common violations include:
Missing signatures or dates
Outdated consent forms used after amendments
Failure to re-consent after protocol revisions
Each error is considered a serious protocol deviation and can lead to participant exclusion from analysis.
Poor Delegation of Duties
GCP requires every staff role to be documented via Delegation of Authority (DOA) logs. Often, untrained team members perform critical tasks — such as drug dispensing or AE documentation — without proper delegation or training logs. This creates gaps that fail audits and invalidate safety reporting.
Incomplete or Inaccurate Source Documentation
Source data must match the case report forms (CRFs) and must be traceable. Pitfalls include:
Backdated entries
Discrepancies between lab reports and CRFs
Missing visit notes or dosing details
These issues trigger data queries and warning letters, especially during FDA or EMA inspections.
Inconsistent Adverse Event Reporting
Failure to report AEs or SAEs within regulatory timelines is a high-risk violation. Problems arise when:
AE logs are maintained separately from the eCRF
Investigators fail to grade or assess relatedness
Sponsors are not notified within the required window
Sponsors are legally bound to report SAEs quickly. Late reporting is one of the most cited violations in inspection reports.
Lack of CAPA Follow-Through
Sites and sponsors often create Corrective and Preventive Action (CAPA) plans after a deviation, but fail to implement them effectively. CAPA without follow-up is viewed as negligence — not effort.
CAPAs must include:
Root cause analysis
Target dates and measurable actions
Documentation of completed steps and training
Without real follow-through, repeated deviations expose systemic quality failures.
Master GCP Through Our Certification Program
The Good Clinical Practice Certification by CCRPS is designed for clinical research professionals who need more than surface-level training. It goes beyond compliance checklists and dives deep into real-world trial execution, regulatory alignment, and global readiness. With over 200+ modules, this self-paced course helps you build mastery in every facet of ICH-GCP — from protocol design to audit response.
Whether you're a CRC, CRA, site manager, or sponsor-side professional, this certification equips you with tools to manage GCP expectations across global regulatory frameworks. It’s not just about knowing the guidelines — it’s about applying them under pressure, during inspections, and in multi-site environments.
You'll leave the course understanding how to set up quality systems, execute CAPA processes, and speak the language of FDA, EMA, and PMDA inspectors. For career advancement, GCP certification isn’t just an asset — it’s a strategic necessity.
What CCRPS Offers
CCRPS is one of the few organizations offering accredited, in-depth GCP training tailored for modern trial environments. Unlike short webinars or outdated PDF-based training, this program includes:
Self-paced modules covering ICH-GCP E6 (R2) and E6 (R3) guidance
Lessons on protocol deviations, safety reporting, eSource compliance, and RBM
Tools for SOP development, monitoring plans, and TMF quality control
Real-world case studies and mock audit scenarios
Quizzes and certification exam aligned with FDA/EMA expectations
The course is mobile-accessible, CPD-accredited, and continuously updated based on current GCP violations observed during real inspections.
Once completed, you'll receive a verifiable certificate demonstrating your readiness to manage high-risk trials and meet sponsor, CRO, and regulatory standards.
Real-World Applications of GCP Training
GCP training isn’t theoretical — it directly impacts your daily operations. Certified professionals report:
Fewer protocol deviations due to stronger risk anticipation
Improved audit outcomes through clear documentation and role delegation
Faster onboarding for global studies with standardized GCP language and procedures
Greater promotion opportunities within CROs, biotech firms, and regulatory agencies
For example, a CRA trained under CCRPS reported resolving a CAPA with a sponsor in 2 days — down from 3 weeks — by applying standardized deviation classification and risk scoring techniques taught in the course.
Mastery of GCP turns you from a task executor into a trial leader. Certification isn’t the end goal — it’s the beginning of operating with precision, foresight, and regulatory fluency.
Frequently Asked Questions
-
Good Clinical Practice (GCP) is an internationally accepted ethical and scientific framework that governs how clinical trials involving human subjects should be conducted. It ensures that the rights, safety, and well-being of participants are protected and that the data generated is credible, accurate, and verifiable. GCP guidelines are mandated by authorities like the FDA, EMA, and ICH, and are universally applicable to pharmaceutical, medical device, and biologics trials. GCP encompasses everything from informed consent to data management and investigator accountability. Compliance with GCP is legally required in most countries and forms the foundation for trial approvals, audits, and eventual product licensure. Without it, clinical data is considered unreliable and may be excluded from regulatory review.
-
GCP training is essential because it teaches professionals how to conduct trials that are both ethically sound and legally compliant. Regulatory agencies expect staff at every trial site — including CRAs, CRCs, investigators, and data managers — to be fully trained and GCP-certified. Without this foundation, sites are prone to protocol violations, improper consent documentation, and data discrepancies. In an increasingly globalized research landscape, GCP compliance is the universal language that ensures consistency across regions. Most sponsors and CROs won’t allow individuals to work on their studies without proof of recent, accredited GCP training. It’s not just about compliance — GCP certification enhances trust and employability in a competitive field.
-
Most sponsors and regulatory bodies recommend that GCP training be renewed every 1 to 3 years, depending on the level of oversight required. While GCP itself doesn’t change frequently, updates like ICH E6 (R3) or FDA guidance revisions require professionals to refresh their knowledge. Some organizations, including sponsors and CROs, may require more frequent renewals to ensure site personnel are aligned with evolving standards. Renewal is especially important when working in high-risk trials, global submissions, or with investigational devices. If you’re switching roles — such as moving from CRC to CRA — renewing your Good Clinical Practice Certification by CCRPS ensures your training reflects your responsibilities.
-
Good Clinical Practice (GCP) is a global framework for ethical and scientific trial conduct, while Standard Operating Procedures (SOPs) are site- or organization-specific documents that define exactly how tasks should be performed. GCP tells you what must be done — such as obtaining informed consent or maintaining accurate source data — while SOPs define how that should be done in your setting. For example, GCP requires accurate AE documentation, but your SOPs will outline which forms to use, where to file them, and how to escalate safety issues. Proper GCP training helps ensure your SOPs are aligned with global compliance standards.
-
Absolutely. GCP certification is a hiring baseline across sponsor, CRO, and site-level roles. Clinical Trial Assistants (CTAs), Coordinators (CRCs), Monitors (CRAs), and Regulatory Specialists are often filtered based on current GCP training status. Certification from a recognized body — especially one like the Good Clinical Practice Certification by CCRPS — signals that you understand not just the rules, but how to apply them. Employers view GCP-certified professionals as less risky, more inspection-ready, and easier to onboard. In fact, many job listings explicitly require documented GCP training completed within the last 12–24 months. It can make the difference between being shortlisted or skipped.
-
Non-compliance with GCP can trigger serious consequences. For starters, regulatory inspections may result in warning letters, FDA 483s, or site disqualification. Common violations include missing consent documentation, data fabrication, or failing to report adverse events. In extreme cases, entire datasets can be excluded from regulatory review, delaying product approvals or even leading to trial shutdowns. Sponsors may terminate contracts with non-compliant sites or investigators, which can damage professional reputations permanently. From a legal standpoint, violations of GCP are considered breaches of federal law in many countries. This is why comprehensive GCP training and strong SOP adherence are essential for every trial site.
-
GCP is primarily designed for interventional trials involving human participants, but its principles still apply — in part — to observational, retrospective, and non-interventional studies. Elements like informed consent, data accuracy, subject confidentiality, and ethical oversight remain relevant. While not always legally required in non-interventional settings, many sponsors voluntarily enforce GCP-level standards across all research types to maintain quality, auditability, and brand integrity. Additionally, if a study involves identifiable health data or biological specimens, GCP-aligned practices help ensure compliance with GDPR, HIPAA, and other privacy laws. Most regulatory-minded institutions err on the side of GCP adherence, even when it’s optional.
-
The Certified Clinical Research Professionals Society (CCRPS) offers one of the most comprehensive, up-to-date GCP programs available. Unlike basic 1-hour courses or generic slide decks, CCRPS’s training includes 200+ lessons covering ICH E6 (R2/R3), FDA guidance, risk-based monitoring, eConsent, audit prep, and more. It’s fully self-paced, CPD-accredited, and designed by active industry professionals. Quizzes and case studies are based on real regulatory findings — not just textbook theory. Whether you’re preparing for an audit, leading a site, or entering the field, the Good Clinical Practice Certification by CCRPS equips you with regulatory fluency and applied confidence.
Final Thoughts
Good Clinical Practice (GCP) is not just a set of guidelines — it’s the operational core of any ethical, regulatory-compliant clinical trial. From protecting human subjects to generating submission-ready data, GCP mastery transforms how trials are conducted, reviewed, and approved. It’s the difference between running a study that merely meets expectations and one that sets the standard.
Whether you're a new coordinator or a seasoned CRA, keeping up with evolving standards like ICH E6 (R3) is no longer optional. Regulatory bodies demand fluency, not familiarity. That’s why targeted, practical, and accredited training matters. With the Good Clinical Practice Certification by CCRPS, you gain more than a credential — you gain readiness to lead high-stakes trials with precision and integrity.
As clinical research becomes more decentralized, global, and digitized, professionals who understand both foundational GCP and its real-world execution will remain in highest demand. Compliance doesn’t slow progress — it accelerates approval.
How confident do you feel about implementing GCP guidelines in your clinical research role?