Data Integrity in Clinical Research: Why It Matters and How to Ensure It
Data integrity is the cornerstone of clinical research. Without reliable, accurate, and consistent data, the entire framework of clinical trials would collapse. Clinical research relies heavily on data to make informed decisions about drug efficacy, safety, and regulatory compliance. If the data collected during these trials is flawed or compromised, it could lead to incorrect conclusions, jeopardizing patient safety, delaying life-saving treatments, and tarnishing the reputation of researchers and organizations alike.
In this blog, we will dive deep into why data integrity is so important in clinical research, the factors that can compromise data integrity, and practical steps to ensure its robustness.
What is Data Integrity in Clinical Research?
Data integrity in clinical research refers to the accuracy, consistency, and reliability of data throughout its lifecycle. From the moment a clinical trial begins to its conclusion, the data must be collected, recorded, analyzed, and stored without alteration, loss, or unauthorized access. Data integrity is crucial in maintaining the credibility of clinical research and ensuring patient safety.
In clinical trials, data integrity directly impacts the decision-making process. For instance, if the trial data is manipulated or incomplete, it could lead to the approval of ineffective or unsafe drugs. This is why regulatory agencies, like the FDA and EMA, have strict guidelines for maintaining data integrity.
To better understand how Electronic Data Capture (EDC) systems enhance data integrity, you can explore our detailed blog.
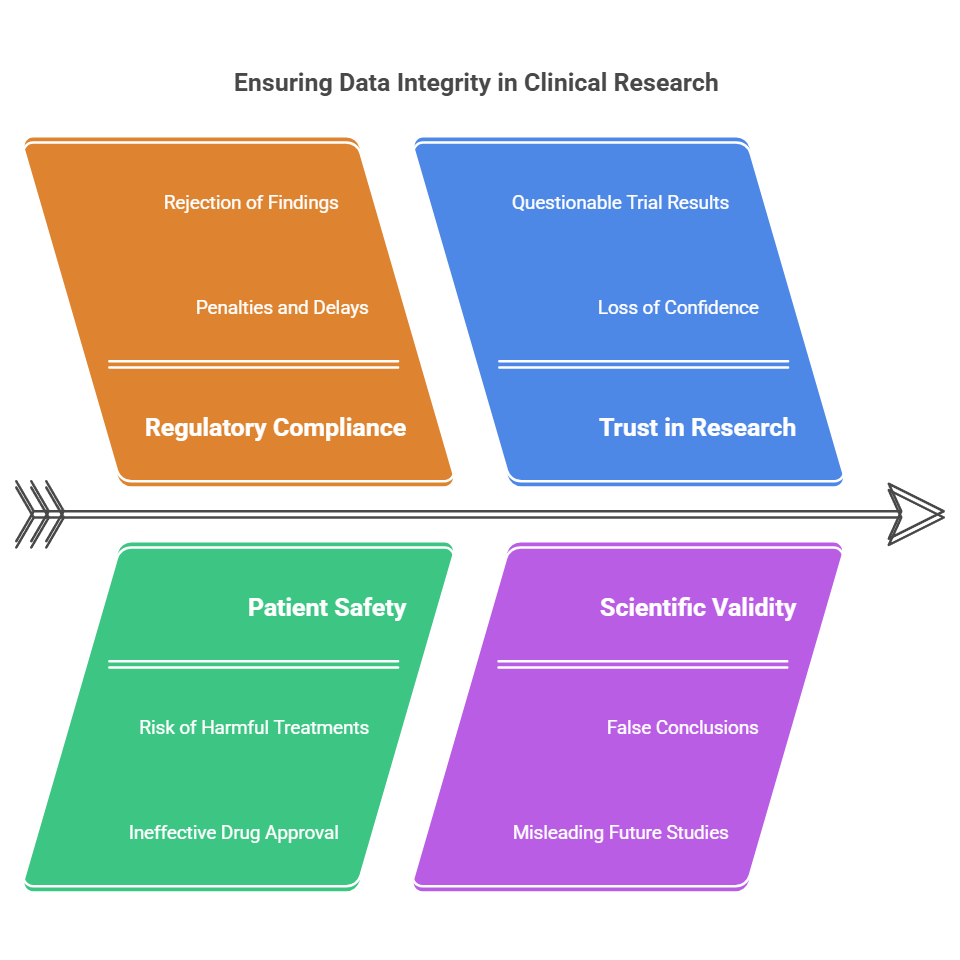
Why Data Integrity Matters in Clinical Research
Regulatory Compliance Regulatory bodies like the FDA, EMA, and ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) have strict guidelines governing clinical trials. They require that data be accurate, complete, and verifiable. Any breach in data integrity could lead to penalties, rejection of research findings, or delays in drug approval processes.
Patient Safety Clinical research often involves human subjects, and the data collected can influence their treatment. Ensuring data integrity safeguards patient safety by ensuring that treatments and interventions are evaluated based on accurate data. Inaccurate data can lead to harmful treatments being administered or ineffective drugs being approved.
Trust in the Research Data integrity helps build trust within the scientific community and the public. Researchers, sponsors, and patients must trust that the data used in clinical trials is reliable. If the data is compromised, the results of the trial are questionable, which undermines the confidence in the research.
Scientific Validity Clinical trials generate data that contributes to the scientific knowledge base. Inaccurate or falsified data leads to false conclusions and can mislead future studies. The advancement of medicine depends on the integrity of the data gathered in clinical research.
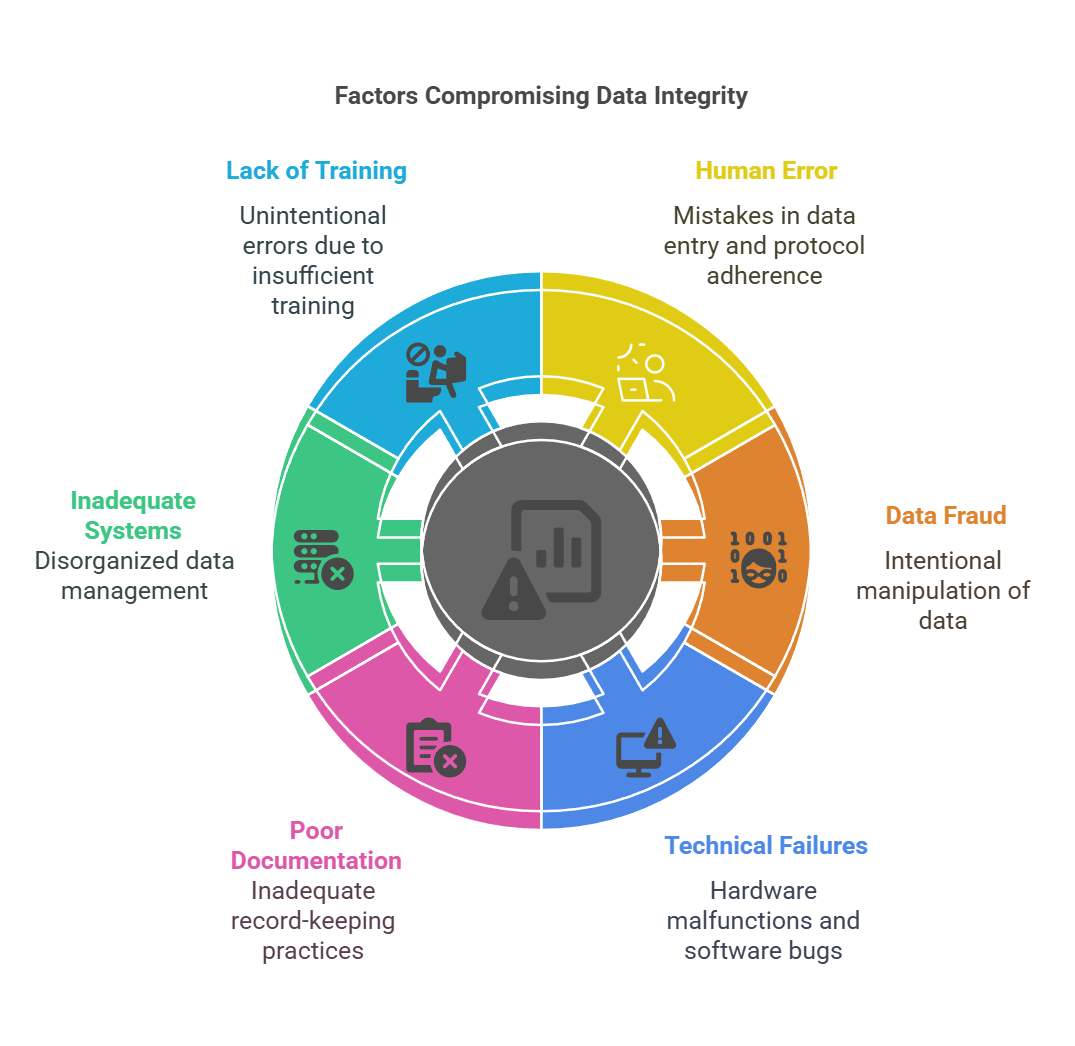
Factors That Compromise Data Integrity
Data integrity is vulnerable to several factors during the lifecycle of clinical research. Understanding these factors can help mitigate risks and ensure high-quality, reliable data.
Human Error Human error is one of the most common threats to data integrity. Simple mistakes such as incorrect data entry, failure to follow protocol, or missing data points can compromise the entire dataset. Human factors such as stress, fatigue, or lack of training can further exacerbate the risk of errors.
Data Fraud or Fabrication Fraudulent data manipulation is an unfortunate reality in some clinical research studies. Whether intentional or not, data fraud compromises the entire study, affecting the safety of participants and the credibility of the results. Regulatory bodies such as the FDA take data falsification very seriously, and researchers caught in fraudulent activities face severe penalties, including professional disqualification.
Technical Failures The reliance on electronic systems for data collection and storage introduces the risk of technical failures. Data loss due to hardware malfunctions, system crashes, or software bugs can result in the destruction or corruption of valuable clinical trial data.
Poor Documentation Practices Inadequate documentation of study protocols, patient consent forms, and other essential trial data can lead to gaps in the record-keeping process, making it difficult to verify the integrity of data. Proper documentation ensures transparency and accountability throughout the trial.
Inadequate Data Management Systems Without robust data management systems, clinical trials are susceptible to inconsistent or incomplete data collection. A poor data management system can lead to disorganized records, making it difficult to track and verify data across sites.
Lack of Training and Education Clinical trial staff must be well-versed in the importance of data integrity and trained in proper data management techniques. A lack of training can lead to unintentional errors or breaches in data integrity. Ensuring all involved personnel are educated on proper data handling protocols is crucial to maintaining data quality.
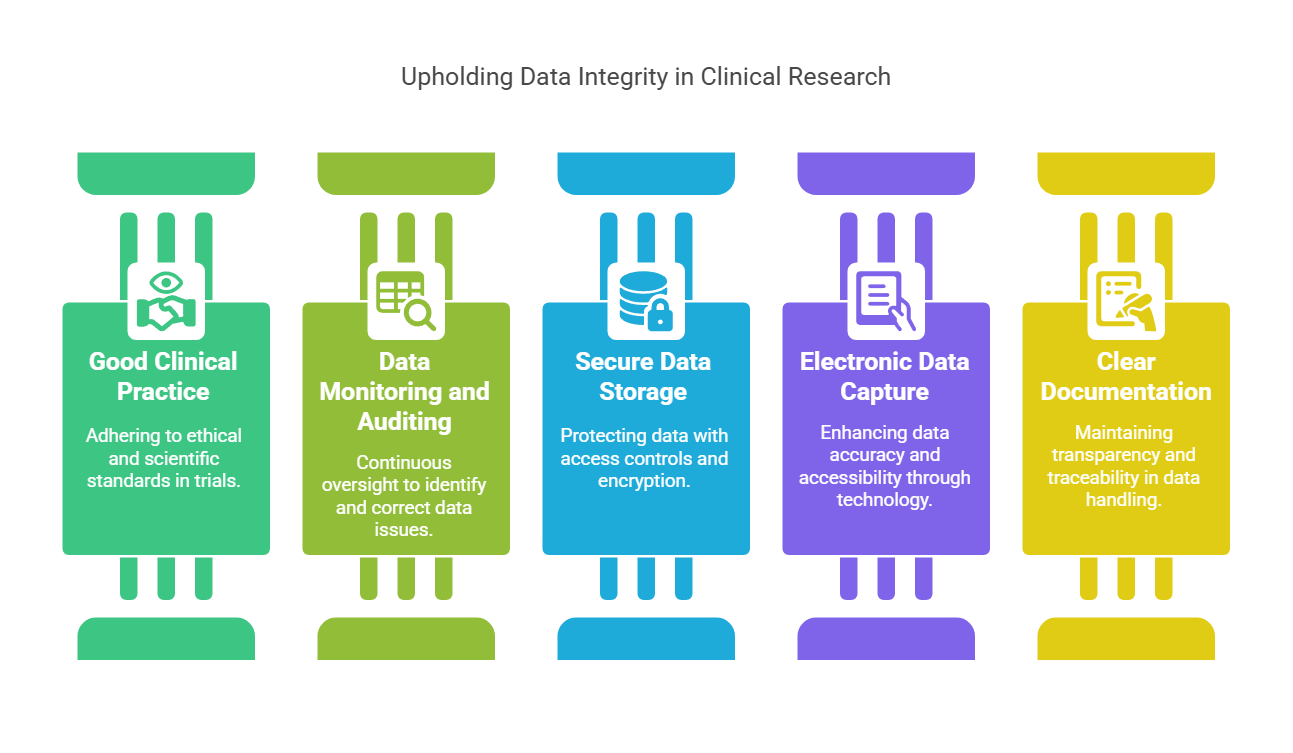
Key Principles for Ensuring Data Integrity
Ensuring data integrity requires a combination of best practices, rigorous monitoring, and adherence to regulatory guidelines. Here are the key principles to uphold data integrity in clinical research:
Good Clinical Practice (GCP) GCP guidelines are internationally recognized standards for the conduct of clinical trials. Adhering to GCP ensures that trials are conducted with the highest ethical and scientific standards, protecting the rights of participants and ensuring data integrity.
Data Monitoring and Auditing Continuous monitoring of clinical trial data helps identify discrepancies and irregularities. Auditing processes ensure that all data points are accurately recorded and that the research adheres to predefined protocols. Regular audits also help identify errors and provide opportunities for corrective action.
Secure Data Storage and Access Control Data security is paramount in maintaining integrity. Secure electronic data capture systems (EDC) should be used to store data, with strict access controls to ensure that only authorized personnel can modify or access the data. Encryption and backup protocols must be in place to safeguard data from unauthorized tampering or loss.
Electronic Data Capture (EDC) Systems Using EDC systems to collect, store, and manage data enhances data integrity by reducing human error, improving data accessibility, and ensuring data security. These systems enable real-time data entry and validation, making it easier to detect discrepancies and correct them promptly.
Clear Documentation Transparent and consistent documentation is vital for maintaining data integrity. Documentation of all procedures, from patient consent to data entry, should be clear and well-maintained. This allows for easy verification of data and ensures that any discrepancies can be traced to their source.
Data Validation Procedures Data validation is the process of ensuring that data is accurate and meets predefined standards. Automatic validation checks in EDC systems help identify errors early in the process, reducing the chances of inaccurate data making its way into the trial’s results.
Regular Training for Personnel Ongoing training for clinical trial staff is essential to ensure they understand the importance of data integrity and are up-to-date with the latest standards, tools, and practices. This helps prevent human errors and ensures consistency in data handling.
Clear Communication Across Sites In multi-site clinical trials, it is essential to maintain clear and consistent communication between all involved parties. Establishing a standardized process for data collection and reporting across all sites ensures that data integrity is maintained across the board.
Pro Tip: If you're interested in gaining in-depth knowledge on data management in clinical trials, our training resources provide comprehensive insights into best practices.
Best Practices for Ensuring Data Integrity
To help clinical research professionals protect data integrity, here are a few best practices to follow:
Implement Data Integrity Policies – Establish clear policies for data collection, handling, and storage to ensure everyone involved is aware of their responsibilities in maintaining data integrity.
Use Secure EDC Systems – Invest in a secure EDC system that provides real-time data validation, audit trails, and encrypted data storage. This helps maintain high data integrity throughout the trial process.
Conduct Regular Training – Provide regular training sessions for all clinical trial personnel on best practices for data management and the importance of maintaining data integrity.
Ensure Consistent Audits and Monitoring – Conduct regular audits of data, monitor for discrepancies, and perform routine checks to verify the accuracy of data collected from all trial sites.
Monitor and Validate Data in Real-Time – Use real-time monitoring to catch discrepancies as soon as they arise. This allows for quick corrective action and ensures that no errors are overlooked.
Create Comprehensive Data Handling Guidelines – Develop clear guidelines for data handling and documentation to prevent inconsistencies and ensure that data can be tracked and verified at any time.
10 Less-Commonly Known Facts
1. Data Integrity Starts at the Study Design Phase
Data integrity isn't just about handling data correctly during the trial. It begins during study design when protocols are written. A study with poor design can lead to inaccurate data collection.
Source: Journal of Clinical Research Best Practices
2. Clinical Trials Can Be Terminated Due to Data Integrity Issues
If significant issues with data integrity are found, regulatory bodies can suspend or terminate a clinical trial. This ensures that misleading results are not presented to the public or regulators.
Source: FDA: Clinical Trial Regulations
3. The Cost of Data Integrity Failures Can Be High
Data integrity issues can lead to the loss of clinical trial data, which in turn may require the entire trial to be repeated, costing millions of dollars and years of research effort.
Source: National Institutes of Health (NIH)
4. Paper-Based Data Is More Prone to Integrity Issues
Paper-based clinical trial records are more susceptible to errors, including loss, misfiling, and difficulty tracking changes. Digital records are far more reliable and easier to secure.
Source: Journal of Clinical Data Management
5. Data Integrity is Regulated by GxP (Good Automated Manufacturing Practice)
GxP regulations ensure that automated systems used in clinical trials for data capture and management maintain data integrity by establishing proper procedures, validation, and security.
Source: European Medicines Agency (EMA)
6. Audit Trails Are Essential for Data Integrity
Audit trails, which track all data modifications, are crucial for maintaining data integrity. These logs are often reviewed by regulatory bodies to ensure no unauthorized changes occurred during the trial.
Source: Clinical Trials.gov
7. Data Integrity Can Be Impaired by Fraudulent Practices
Fraudulent activities, such as falsifying data to support a desired outcome, can severely impact data integrity and compromise the entire study’s validity.
Source: Journal of Clinical Research Fraud Prevention
8. Electronic Data Capture (EDC) Systems Enhance Data Integrity
EDC systems help minimize human error and streamline data collection, ensuring that data is accurately entered and securely stored, contributing to better data integrity.
Source: Clinical Research Society
9. Data Integrity is Linked to Patient Safety
Poor data integrity may lead to false conclusions about the safety and efficacy of a drug or treatment, potentially putting patients at risk in subsequent phases of research.
Source: World Health Organization (WHO)
10. Data Integrity Can Be Ensured with Regular Monitoring and Training
Regular monitoring of clinical data and ongoing staff training in data management techniques significantly reduce the risk of data integrity issues in clinical trials.
Source: Clinical Trial Management Institute
Conclusion
Data integrity is essential to the success of clinical trials and the safety of participants. By adhering to best practices, implementing rigorous monitoring systems, and using secure EDC systems, clinical research professionals can ensure that data remains accurate, consistent, and reliable. This not only helps meet regulatory requirements but also builds trust in the research process, ultimately benefiting patients and advancing medical knowledge.
If you want to learn more about maintaining data integrity in clinical research or explore other topics related to clinical research training and certification, visit CCRPS for valuable resources and certifications that can advance your career.
-
Data integrity in clinical research refers to the accuracy, consistency, and reliability of data collected, stored, and analyzed throughout the trial. It ensures the data is unaltered and trustworthy.
-
Data integrity ensures that clinical trial results are accurate and reliable, which is vital for patient safety, regulatory compliance, and the credibility of the research findings.
-
Common threats include human error, data fraud, technical failures, poor documentation practices, and inadequate data management systems.
-
Human error can occur through mistakes like incorrect data entry, missed information, or failure to follow study protocols, which can lead to inaccurate or incomplete data.
-
An EDC system is a digital tool used to collect, store, and manage clinical trial data, helping improve accuracy, streamline data entry, and enhance data security.
-
By adhering to Good Clinical Practices (GCP), using secure EDC systems, conducting regular audits, and training staff in data management, clinical trials can maintain data integrity.
-
GCP is a set of internationally recognized standards for designing, conducting, and reporting clinical trials. It ensures that trials are ethical, scientifically sound, and that data integrity is upheld.
-
Proper documentation of study protocols, data collection methods, and consent forms helps ensure transparency and accountability, making it easier to verify data integrity throughout the trial.