Future Trends in Clinical Trials: What Clinical Research Coordinators Should Prepare For?
The clinical trial environment is undergoing substantial changes. The upcoming 2025 period requires Clinical Research Coordinators (CRCs) to understand new trends which will help them execute and monitor clinical studies effectively. The field has witnessed three major developments which include digital transformation and artificial intelligence (AI) integration and changes in regulatory requirements. This article examines these trends while providing CRCs with necessary guidance to succeed in future clinical trials.
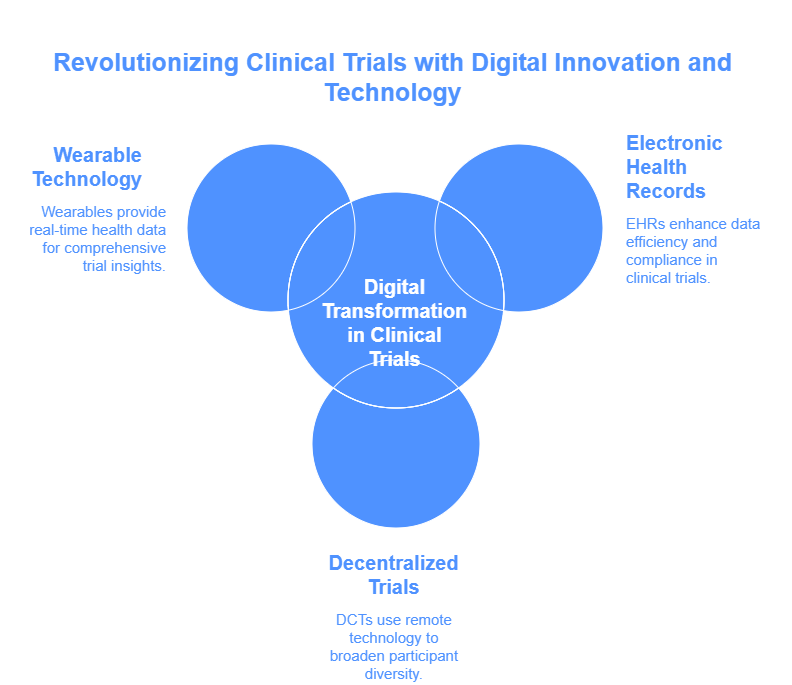
Digital Transformation in Clinical Trials
Adoption of Electronic Health Records (EHRs)
The large-scale adoption of Electronic Health Records (EHRs) has transformed the process of collecting and managing data in clinical trials. EHRs enable researchers to easily retrieve patient information which leads to improved clinical research efficiency and data accuracy. CRCs need to demonstrate EHR system proficiency because it ensures both proper data management and privacy regulation compliance.
Decentralized Clinical Trials (DCTs)
The adoption of Decentralized Clinical Trials (DCTs) has accelerated since the COVID-19 pandemic. The implementation of telemedicine together with mobile health devices and remote monitoring enables DCTs to eliminate the requirement of participants visiting central trial sites. The method enhances participant diversity while simultaneously leading to better participant retention. CRCs need to build expertise in handling remote communication while maintaining data accuracy within virtual environments.
Wearable Technology and Mobile Health Apps
Real-time data collection through wearable devices and mobile health applications allows researchers to monitor participants' health metrics continuously. The technology delivers an enhanced understanding of treatment effects together with patient well-being. CRCs need to demonstrate skill in incorporating device data into trial operations while managing security concerns and participant data adherence.
Integration of Artificial Intelligence in Clinical Trials
AI-Driven Patient Recruitment
Artificial intelligence has transformed patient recruitment by analyzing vast datasets to identify suitable candidates swiftly. AI algorithms can predict patient eligibility based on medical histories, demographics, and genetic information, thereby reducing recruitment timelines and enhancing trial efficiency. CRCs should familiarize themselves with AI tools to optimize recruitment strategies.
Predictive Analytics for Trial Design
AI-powered predictive analytics assist in designing more effective clinical trials by forecasting potential outcomes and identifying risks. This approach allows for adaptive trial designs that can be modified based on interim results, improving success rates and resource utilization. Understanding these analytics enables CRCs to contribute to more dynamic and responsive trial protocols.
Automation in Data Management
Automation through AI streamlines data collection, cleaning, and analysis processes. By minimizing manual errors and expediting data processing, AI enhances the reliability of trial results. CRCs should embrace training in AI-driven data management systems to oversee these processes effectively.
Evolving Regulatory Frameworks
Global Harmonization of Regulations
The globalization of clinical research necessitates harmonized regulatory standards to facilitate multi-regional trials. Initiatives like the International Council for Harmonisation (ICH) work towards standardizing guidelines across countries, simplifying approval processes, and ensuring consistent quality. CRCs must stay informed about these developments to navigate international regulatory landscapes proficiently.
Emphasis on Diversity and Inclusion
Regulatory bodies are increasingly mandating the inclusion of diverse populations in clinical trials to ensure that findings are generalizable across different demographics. This shift requires CRCs to implement strategies that promote the enrollment of underrepresented groups, addressing barriers such as language, cultural differences, and accessibility.
Data Privacy and Security Regulations
With the rise of digital data collection, stringent data privacy and security regulations have been enacted to protect participant information. Compliance with laws such as the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA) is critical. CRCs must ensure that data handling practices meet these standards to maintain participant trust and legal compliance.
Lesser-Known Facts About Future Trends in Clinical Trials
AI-Generated Digital Twins: Researchers are developing AI-generated digital twins to simulate patient responses, potentially reducing the need for large control groups in trials. This technology enhances trial efficiency and personalizes treatment predictions. (Source)
Blockchain for Data Integrity: Blockchain technology is being explored to enhance data integrity and transparency in clinical trials, ensuring immutable and verifiable records. While specific references are not provided, blockchain's potential in clinical trials is increasingly recognized for its ability to secure data. (Source)
Virtual Reality (VR) in Patient Engagement: VR is being utilized to improve patient engagement and adherence by providing immersive educational experiences about the trial process. This approach aims to enhance patient understanding and participation.
Real-Time Data Sharing Platforms: Emerging platforms enable real-time data sharing among stakeholders, facilitating quicker decision-making and adaptive trial designs. These platforms leverage AI and machine learning to optimize trial processes. (Source)
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
The future of clinical trials is marked by rapid technological advancements and shifting regulatory paradigms. For Clinical Research Coordinators, staying informed and adaptable is key to navigating these changes effectively. By embracing digital transformation, leveraging AI innovations, and adhering to evolving regulations, CRCs can enhance the efficiency and quality of clinical trials. Organizations like CCRPS provide valuable resources and training to support CRCs in this journey, ensuring they are well-equipped to meet the challenges and opportunities that lie ahead.
Frequently Asked Questions (FAQs)
-
AI analyzes large datasets to identify potential trial participants who meet specific eligibility criteria, thereby streamlining the recruitment process and reducing timelines.
-
Decentralized trials offer participants the convenience of participating from their homes, utilizing telemedicine and remote monitoring, which can increase accessibility and retention.
-
Synthetic control arms use historical data and AI models to create virtual control groups, potentially reducing the need for actual participants to receive place.