Introduction to Clinical Trial Training
Hold onto your lab coats and grab a beaker of curiosity—welcome to the quirky universe of clinical trial training! Imagine a world where every pill, potion, and therapy is a tiny superhero waiting to be unleashed. But before they can save lives, they must pass the ultimate test: clinical trials. Intrigued? Buckle up as we dive into the meticulous yet exhilarating journey from lab bench to bedside!
Have you ever wondered how new medications and treatments make it from the lab to your doctor's office? The answer lies in clinical trials, meticulously designed research studies that test the safety and effectiveness of new healthcare interventions on human volunteers.
If you are interested in this field and if you are planning to build your career in clinical research, an introduction to clinical trial training could not be a bad place to start. This blog post will provide you with the basic information that will help you start your journey.
Why the Rise in Clinical Research Training?
Why are there all of a sudden more wizards of science, I mean clinical researchers, popping up everywhere? The realms of pharmaceuticals and biotech are evolving faster than a speeding placebo and the demand for these skilled professionals has skyrocketed. They are the unsung heroes who make sure that new treatments are not only revolutionary but also safe.
Clinical Research Associates: The Overseeing Force
A clinical research associate (CRA) is an important component of the clinical trial process. They are the eyes and ears of the sponsor, and they monitor the subject’s care and and make sure that the study follows the rules. They are involved in activities such as monitoring investigator sites, making sure that data is accurate and complete, and reporting any problems or discrepancies with the protocol. If you are interested in learning more about becoming a CRA, then this specialized CRA training course might be of interest to you.
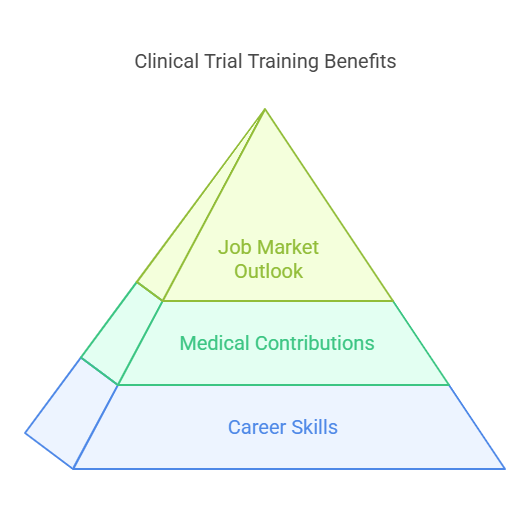
Benefits of Clinical Trial Training
With the clinical research market projected for continued growth, a thorough understanding of clinical research training is essential. Here's how it benefits you:
Equipping Yourself for a Rewarding Career: This training will provide the skills and knowledge needed to get a successful clinical research career off the ground. You can tailor your path to your interests because there are various specializations within the field.
Contributing to Medical Advancements: Through clinical trials you contribute to the development of new treatments and hence patient care. You are therefore enabled by the knowledge obtained through training to be of great value in this endeavor.
Strong Job Market Outlook: The need for trained clinical research professionals is high and expected to rise even higher. This training prepares you to capitalize on this expanding market demand.
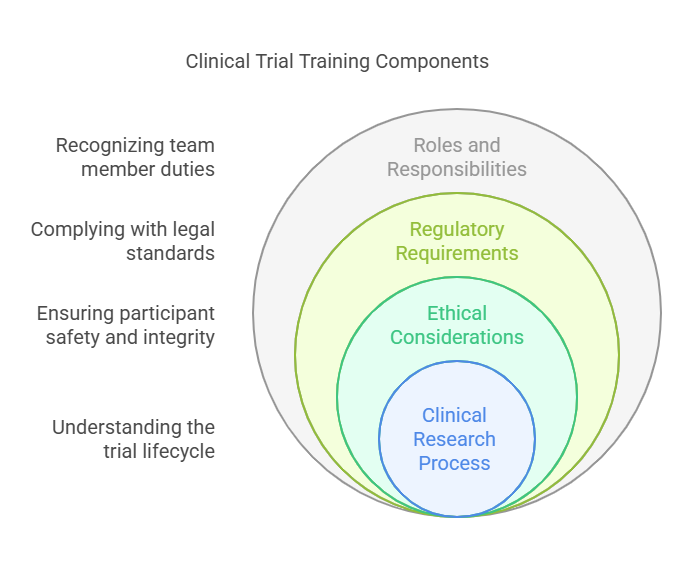
What Does Clinical Trial Training Entail?
Clinical trial training equips individuals with the necessary understanding of:
The Clinical Research Process: You’ll get insights into the full clinical trial lifecycle – from study design and participant recruitment, to data collection and analysis.
Ethical Considerations: Above all, participant safety and well-being must be protected. Training focuses on informed consent, patient confidentiality and responsible research conduct.
Regulatory Requirements: Clinical trials are governed by strict rules and regulations of regulatory bodies. You need to be trained to understand these regulations and to be able to use them properly.
Specific Roles and Responsibilities: A clinical research team is composed of various professionals, with each having different responsibilities. It is important to know your role during training and how it contributes to the success of the trial.
Who Should Consider Clinical Trial Training?
This training is valuable for anyone interested in a career in clinical research, including:
Clinical research coordinators (Consider the Clinical Research Coordinator course)
Data managers
Clinical trial assistants (Explore the Clinical Trials Assistant Training)
Regulatory affairs specialists
Research nurses
Physicians interested in research (Check out the Advanced Principal Investigator Physician Certification)
Where to Find Clinical Trial Training?
There are numerous avenues to pursue clinical trial training. Here are some options to consider:
Online Courses: Several organizations offer introductory and advanced online courses in clinical research, including Pharmacovigilance Certification and ICH-GCP.
Universities and Colleges: Many academic institutions offer certificate programs or degrees in clinical research
Professional Associations: Clinical research professionals are often trained through the workshops and training programs that are usually hosted by industry associations.
Begin Your Clinical Research Journey
In this way, by taking an introduction to clinical trial training you will uncover many opportunities in healthcare improvement. You will be able to identify the key principles that will help you to make the right decision about your future career in this fast changing environment.
Remember, this is just the beginning! As you delve deeper, you'll discover the profound impact clinical trials have on improving human health and shaping the future of medicine.
I. Clinical Research Training: Background and Importance
It is important to have some background on what clinical research is, and what role it plays in the medical field before looking at the details of the clinical research training. The term clinical research is the study of a large number of potential new drugs, medical devices and techniques to find out if they are safe and effective and therefore able to be approved by regulatory authorities. In its most basic form, clinical research is the process of determining whether new treatments and therapies are not only safe for human use, but also effective – and thus protecting the public from potential harmful side effects or complications.
This means that clinical research has a vital role in the process of taking new medical treatments to the market. It is a broad process that entails a number of skills from data analysis to ethics and compliance. Clinical research associates (CRAs) supervise clinical trials and monitor that they are conducted efficiently and in compliance with the laws and ethical norms. Therefore, clinical research training provides the necessary skills to potential CRAs to perform their duties efficiently and help in the development of new therapies and medical products safely.
II. Components of Clinical Research Training
Clinical research training typically comprises several essential components, each designed to provide a comprehensive understanding of the clinical research process. Some of the critical elements of clinical research training include the following:
Basic Principles of Clinical Research: A review of the basics of clinical research including clinical trials phases, randomization, blinding and placebo controls.
Good Clinical Practice (GCP): A thorough understanding of GCP guidelines set by regulatory authorities like the International Council for Harmonisation (ICH) and the Food and Drug Administration (FDA) to ensure the safety, integrity, and quality of clinical trials.
Protocol Development: Training in the design and development of clinical trial protocols, including specifying the kind of studies to be done, who is to be included or excluded, and what kind of assessments are needed.
Ethics in Clinical Research: A more in depth look at ethical considerations in clinical research which includes informed consent, institutional review board (IRB) approval, and data protection.
Regulatory Compliance: A comprehensive understanding of the role of various regulatory authorities in the clinical research process and compliance with relevant regulations.
Data Management and Biostatistics: Fundamental knowledge of data management methods, data collection and validation, data quality control and application of biostatistics in clinical research.
Clinical Trial Management: Training in roles and responsibilities of the clinical trial team, and best practices in trial management: site selection, patient recruitment, study closeout.
Safety Reporting and Pharmacovigilance: An understanding of safety reporting requirements and the importance of pharmacovigilance in maintaining patient safety throughout the clinical trial.
III. Clinical Research Training: Course Options and Certifications
For those planning to start or grow in the clinical research industry, many clinical research training programs are available. These programs are usually available to individuals with all kinds of educational backgrounds and all levels of experience, so everyone who wants to be a CRAs can get the training they need. Courses are usually ranging from short workshops to full length diploma or degree programs.
One of the most popular and well-known accreditations is the Clinical Research Associate (CRA) Certification. This means that being certified for this accreditation is a way of showing commitment and professionalism in clinical research. Several organizations provide the clinical research associate certification online, so it is an easy way to learn for many people.
In conclusion, it is important to take clinical research training if one wishes to be involved in clinical research. It enables the learner to acquire skills and information that will enable them to perform and oversee clinical trials and thus protect the public and create opportunities for new therapies. It is therefore possible to obtain the necessary qualifications from the various course options available, including the Clinical Research Associate Certification Online at CCRPS.
Frequently Asked Questions (FAQs)
-
Clinical trial training is an educational pathway focusing on the methodologies and regulations involved in conducting clinical trials to ensure new medical treatments are safe and effective.
-
It is crucial for maintaining the integrity of medical research, ensuring patient safety, and upholding scientific standards that lead to effective healthcare solutions.
-
The duration can vary from a few months to over a year, depending on the depth of the program and whether it's part-time or full-time.
-
Yes, many reputable institutions offer online clinical trial training, allowing you to learn at your own pace and convenience.
-
Graduates can work as clinical research coordinators, clinical research associates, data managers, or in regulatory affairs.
-
Typically, a background in life sciences or related fields is recommended, although many programs are accessible to those with broader academic backgrounds.
-
Absolutely, it's highly beneficial for doctors, nurses, and other healthcare professionals interested in expanding their roles into clinical research.
-
The Clinical Research Associate (CRA) Certification is highly valued, enhancing your credibility and career prospects in clinical research.
-
Unlike general medical training, clinical trial training is specifically tailored to understanding the complexities of trial design, execution, and compliance with global regulatory standards.
-
Absolutely, it significantly enhances your qualifications and makes you a desirable candidate for roles in the burgeoning field of clinical research.
-
You'll acquire skills in project management, regulatory compliance, ethical considerations, and patient interaction—all critical for a successful career in clinical research.
-
Trained professionals ensure that clinical trials are conducted ethically and effectively, directly impacting the development of safer, more effective medical treatments.
-
Many institutions offer scholarships or financial aid to help cover the costs of clinical trial training, making it more accessible to a wider range of students.
-
Trainees often deal with complex regulations, high expectations for accuracy, and the ethical dilemmas of patient interaction in high-stakes environments.
-
Advances in digital tools and data management systems are revolutionizing how clinical trials are taught, with a greater emphasis on real-time data processing and remote monitoring.
-
Clinical trials can often lead to unexpected discoveries about medications, including new uses for existing drugs, which can be just as exciting as developing new therapies!