Why Good Clinical Practice Training is Essential for Clinical Trials
Medical science progresses through clinical trials which lead to better patient results. The success of clinical trials depends on maintaining high integrity and safety and accuracy throughout their conduct. Good Clinical Practice (GCP) stands as the most vital element which supports both the credibility and reliability of clinical trials. This blog examines the necessity of GCP training for clinical trials and its role in maintaining trial integrity and patient safety and regulatory compliance.
What is Good Clinical Practice (GCP)?
Good Clinical Practice (GCP) is an international standard that ensures clinical trials are conducted according to the highest ethical and scientific quality standards. It provides guidelines for the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials. GCP safeguards the rights, safety, and well-being of trial participants, which is crucial in the medical research field.
Related Blog: What Is Good Clinical Practice
Role of GCP in Ensuring Trial Integrity
The integrity of clinical trials remains essential to produce reliable and accurate data throughout the research process. The GCP guidelines implement multiple practices which ensure:
Accurate Data Collection: GCP ensures that data is collected in a consistent and accurate manner. Researchers are trained on proper methods of recording, analyzing, and reporting data to avoid discrepancies.
Minimized Bias: GCP helps to reduce bias by ensuring that trial processes are standardized and that transparency is encouraged. The methods of research and the reporting of outcomes are controlled to prevent the manipulation of results.
Objective Results: The integrity of clinical trials is maintained by ensuring that research outcomes are unbiased and scientifically valid. GCP training helps investigators to avoid misreporting results or engaging in fraudulent practices.
The implementation of standardized methods together with rigorous documentation practices through GCP creates conditions for clinical research integrity and trust which results in more accurate and reproducible results.
Importance of Compliance with Regulatory Bodies (FDA, EMA)
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) regulate clinical trials through strict oversight. The GCP training system enables clinical trials to fulfill the regulatory standards established by these agencies. GCP compliance serves this purpose in the following way:
FDA and EMA Regulations: GCP helps clinical trials comply with essential regulations imposed by bodies like the FDA and EMA. These agencies have specific guidelines regarding trial designs, documentation, patient consent, and safety monitoring, which are all part of GCP standards.
Audit and Inspection Readiness: The professionals trained by GCP know the regulatory framework and make sure that the trial is documented and evidenced to the highest standards to avoid any violations during audits.
Global Harmonization: The GCP guidelines are internationally recognized and help to standardize trials across different regions. This is particularly important for multinational trials as it ensures compliance with different regulatory bodies and minimizes regulatory risk.
The acceptance of clinical trials by regulatory agencies depends on GCP compliance which enables new drugs and medical devices to reach the market.
Related Blog: Top Benefits of GCP Training for Healthcare Professionals
How GCP Training Reduces Errors and Increases Accuracy
The clinical trial process includes multiple intricate steps starting from participant enrollment through data evaluation and small mistakes throughout these stages can create major problems. The following benefits of GCP training minimize errors while improving trial result accuracy:
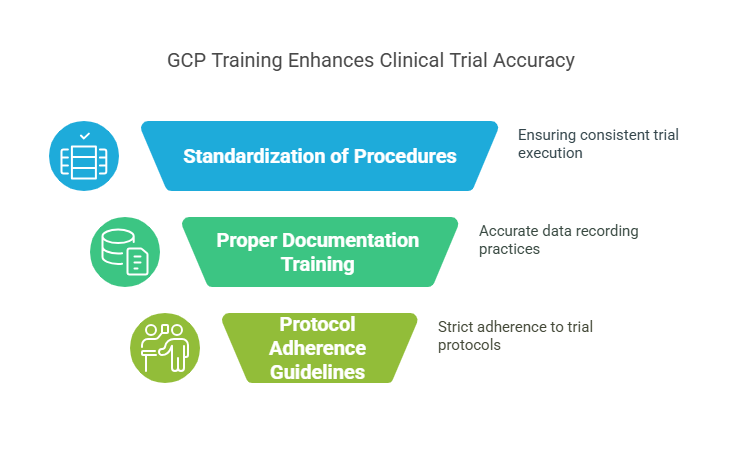
Standardization of Procedures: GCP provides a detailed framework for every aspect of a clinical trial, from protocols to patient interactions. This ensures that all participants, regardless of location, follow the same procedures and reduce variations in results.
Training in Proper Documentation: The main element of GCP involves proper documentation of all activities. The correct training of personnel leads to proper documentation of all data which minimizes errors in data entry and patient information.
Clear Guidelines for Protocol Adherence: GCP requires researchers to follow established protocols exactly as defined to maintain the study's objectives. The study's objectives remain accurate because this approach prevents researchers from deviating from the established procedures.
GCP ensures the reliability and accuracy of clinical trials by minimizing errors and standardizing data collection procedures.
Impact of GCP on Patient Safety
Patient safety stands as a primary focus in clinical trials because these studies require testing new medications and treatments and medical devices whose safety risks remain untested. GCP training ensures that:
Informed Consent: The GCP requires that all participants in clinical trials must give informed consent before they can participate. This means that patients are fully aware of the potential risks, benefits and procedures before they agree to participate.
Adverse Event Reporting: The GCP provides specific guidelines for the monitoring and reporting of adverse events or side effects. It is important to detect adverse events early for the safety of the patient and for the modification of the trial protocol if necessary.
Ongoing Monitoring: The GCP requires the continuous monitoring of patient health throughout the trial. This allows for immediate intervention if any patient experiences harm, thus protecting their well-being.
The GCP training program stresses the significance of patient safety and defines the duties of clinical trial staff members in protecting participants from harm during the entire study period.
Legal and Ethical Considerations in Clinical Trials
The conduct of clinical trials must adhere to strict ethical and legal frameworks which safeguard both research participants and investigators. The training program for GCP covers the following legal and ethical aspects:
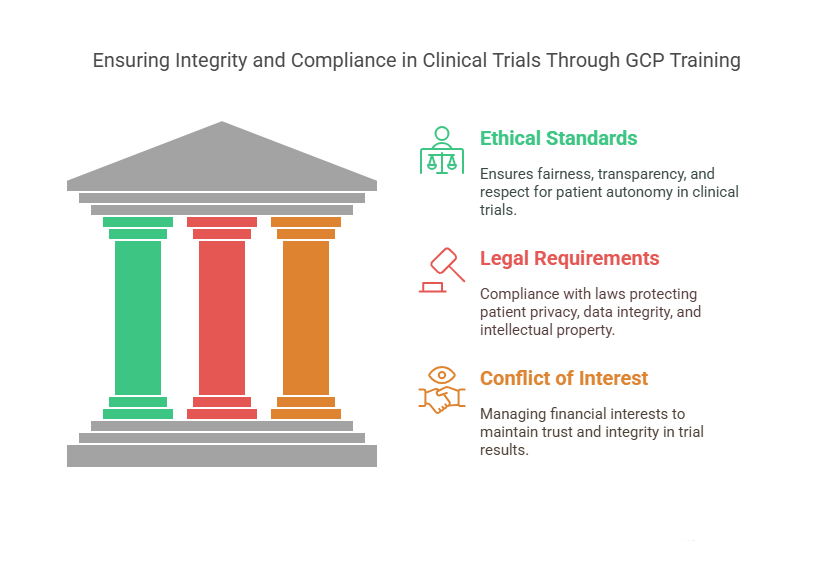
Ethical Standards: GCP ensures that clinical trials are designed with the highest ethical standards including fairness, transparency and respect for patient autonomy. Trials must not include any form of coercion or undue influence on participants.
Legal Requirements: The researchers are trained to ensure compliance with various legal aspects such as patient privacy protection (HIPAA in the U.S.), data integrity, and intellectual property rights. Non-compliance with these regulations can lead to lawsuits, fines, or the invalidation of the study.
Conflict of Interest: The GCP training program stresses the need to control conflicts of interest because they can affect trial results. The study requires proper disclosure and transparency of financial interests to maintain trust in its results.
The GCP framework addresses both legal and ethical aspects to guarantee responsible and ethical clinical trials that protect participants and researchers.
10 Lesser-Known Facts About GCP in Clinical Trials
GCP guidelines are derived from the International Conference on Harmonisation (ICH), an effort to standardize clinical trial practices across the globe. (Source)
The first official GCP guidelines were issued in 1996, marking a significant milestone in clinical trial conduct. (Source)
GCP training is a requirement for professionals involved in clinical trials, including investigators, clinical research coordinators, and monitors. (Source)
GCP compliance can significantly reduce the risk of clinical trial data being rejected by regulatory bodies. (Source)
Clinical trials following GCP guidelines have a higher success rate in gaining regulatory approval from agencies like the FDA. (Source)
GCP involves strict protocols for handling and storing sensitive data to prevent data breaches and ensure patient privacy. (Source)
GCP guidelines are applicable across all phases of clinical trials, from Phase I through Phase IV.
The U.S. FDA has a detailed set of guidelines on GCP and enforces compliance during trial inspections.
Clinical trial auditors specifically look for GCP violations during regulatory inspections, which could halt trial progress.
GCP guidelines also include risk-based monitoring, which ensures that trials focus on areas with the highest potential for risk to participants.
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
In conclusion, Good Clinical Practice (GCP) training is essential for the success of clinical trials. It ensures that trials are conducted with integrity, compliance with regulatory bodies like the FDA and EMA, and a strong focus on patient safety. Moreover, it reduces errors, increases accuracy, and upholds ethical and legal standards, which are critical in producing reliable and trustworthy trial results. For clinical trial professionals, GCP training is not just a regulatory requirement, but a vital tool for ensuring the quality and safety of clinical research. CCRPS is committed to providing top-notch GCP certification and training to professionals, helping them navigate the complexities of clinical trials and contribute to advancing healthcare research.
-
GCP ensures that clinical trials are conducted with integrity, accuracy, and safety, protecting patient rights and ensuring compliance with regulatory standards.
-
Compliance ensures that trials meet legal and ethical standards, and that the results are accepted by regulatory authorities for drug approval and patient safety.
-
GCP standardizes procedures, ensures accurate documentation, and helps prevent protocol deviations, reducing errors and improving data accuracy.
-
GCP mandates informed consent, continuous monitoring, and early detection of adverse events to protect patient well-being during trials.
-
GCP training ensures that trials adhere to ethical guidelines, respect patient autonomy, and comply with legal requirements like data protection and patient privacy.