Norway Clinical Research Career Guide
Ever wondered if Vikings were good at clinical trials? Probably not, but today's Norwegians are conquering new frontiers in clinical research, and you can too! In this comprehensive guide, we delve into the bustling world of Clinical Research Associates (CRAs) in Norway, exploring every corner from salary scales to career pathways that will not only spark your interest but potentially steer your career towards exciting horizons. Join us on this epic journey into the realm of Norwegian clinical research in 2025!
The Role and Value of Clinical Research Associates in Norway
The role of Clinical Research Associates (CRAs) in Norway is crucial and multifaceted, making them central figures in the healthcare and pharmaceutical industries. Let's break down their responsibilities and the unique aspects of their role in the context of Norway's clinical research landscape:
Ensuring Smooth Operation of Clinical Trials: CRAs are the primary managers of clinical trials and supervisory control of their progress and integrity. It implies watching over all aspects of the trials to guarantee they are conducted properly and in accordance with the set rules and regulations and ethical practices. Norway is a country with a wide variety of clinical trials, from the small-scale local studies to the large international collaborations, and the role of a CRA is crucial in ensuring that these studies are accurate and reliable.
Navigating Diverse Environments: Being a Scandinavian nation, Norway’s geographical diversity, which has icy fjords in the north and bustling cities in the south, poses specific challenges and opportunities for clinical research. CRAs must adapt to a number of environmental factors that may have implications for the conduct of clinical trials. This adaptability is crucial whether it’s the remote and rugged terrains of the north or the more urbanized and densely populated areas in the south.
Role in Drug Development: CRAs are involved in the development of new treatments. They play a very important role in the accuracy and completeness of data collected during the trials, which is vital for the drug development process. It is their work that assists pharmaceutical companies and researchers to learn about the efficacy and safety of new treatments before they are available to the public.
Safety and Efficacy: A large scope of the CRA’s work is to protect the health and welfare of the participants in the clinical trials. They watch for and document any adverse events or problems that occur during the course of the trial, so that any new treatment is not only effective but also safe to use in a general population.
Travel and Exploration: The job of a CRA in Norway can involve significant travelling; not only within the country but if the clinical trials are part of global studies, potentially internationally as well. This part of the role is particularly interesting to those who are looking for a career that offers more than just a traditional office environment. It combines science with exploration and travel, making for a dynamic and engaging work life.
Making a Difference: The efforts of Clinical Research Associates (CRAs) make an impact, on the progress of research and the well being of the general public. By overseeing the ethical execution of trials CRAs directly contribute to the creation of therapies that enhance or even save lives. This facet of their profession brings satisfaction as it enables them to witness firsthand the influence their contributions have on communities.
In summary, the role of a Clinical Research Associate in Norway is a blend of rigorous scientific method, adventurous fieldwork, and ethical vigilance. It's a career that offers the chance to be at the forefront of medical research and innovation, with the added benefit of exploring one of the most beautiful and diverse countries in the world. For those passionate about science and looking to make a real impact in the field of medicine, becoming a CRA in Norway could be an ideal career path.
Salary Insights: The Norwegian CRA Market in 2025
In 2025, the salary structure for Clinical Research Associates (CRAs) in Norway reflects both the country's high standard of living and the critical role these professionals play in the clinical research industry. Here's a detailed breakdown of the salary insights for CRAs in Norway:
High Standard of Living: Norway is known for its high quality of life, which is mirrored in the salary levels across various professions, including clinical research. The compensation for CRAs is designed to align with the overall cost of living, which is relatively high in Norway. This ensures that professionals in this field are able to maintain a comfortable standard of living while pursuing their careers.
Salary Ranges Based on Experience:
Entry-Level Positions: For those just beginning their careers as CRAs, the starting salary is around 357,000 NOK annually. This entry-level wage is competitive, providing a solid foundation for new professionals in the field. It reflects the initial responsibilities of monitoring clinical trials, ensuring adherence to protocols, and maintaining accurate data.
Mid-Level Experience: As CRAs gain more experience, particularly in the range of 2 to 10 years, their salaries see significant increases. This progression is due to the accumulation of expertise, deeper understanding of complex clinical trial protocols, and enhanced skills in managing broader aspects of research projects.
Experienced Professionals: For those with extensive experience and specialized skills, particularly those who take on senior roles or manage multiple or international trials, salaries can soar up to 1,090,000 NOK annually. This upper salary range rewards the high level of expertise, leadership, and the significant responsibility they bear in ensuring the success and integrity of clinical trials.
Reflecting Responsibilities: The salary structure is also a reflection of the significant responsibilities that CRAs hold. These include ensuring compliance with regulatory requirements, safeguarding participant safety, managing vast amounts of trial data, and contributing to critical decisions in the development of new therapies. Each of these tasks requires a high level of professional competency, which is rewarded with higher pay as CRAs progress in their careers.
Expertise Pays Off: In Norway, like in many other fields, there is a clear correlation between expertise and compensation. Advanced knowledge and specialized skills in clinical research not only enhance a CRA's ability to contribute to medical advancements but also boost their earning potential. This is especially true as CRAs take on more complex roles, such as Clinical Trial Manager or Clinical Research Coordinator, which come with heightened responsibilities and hence higher compensation.
In conclusion, the salary structure for CRAs in Norway in 2025 is designed to compensate for the demanding nature of the job and the high cost of living, while also providing incentives for professional development and advancement. This approach ensures that the field attracts and retains skilled professionals capable of supporting the country's ambitious clinical research goals.
Link: https://worldsalaries.com/average-clinical-research-associate-salary-in-norway/
Education and Earning Power
In Norway, the field of clinical research is both competitive and highly specialized, with education playing a critical role in determining a Clinical Research Associate's (CRA) career prospects and earning potential. Here’s a deeper look at how educational qualifications impact a CRA’s position in the job market and their salary:
Bachelor’s Degree as a Foundation:
A bachelor's degree in life sciences, such as biology, pharmacy, nursing, or related fields, is the minimum educational requirement to enter the field of clinical research as a CRA. This degree provides the fundamental knowledge of biological and health sciences necessary to understand the medical and technical aspects of clinical trials.
Advantages of a Master’s Degree:
Holding a master's degree in a relevant field, such as clinical research, public health, or biomedical sciences, confers a significant advantage in the job market. This advanced degree not only deepens a professional's understanding of the subject matter but also equips them with specialized skills in research methodologies, statistical analysis, and regulatory compliance.
Master’s degree holders are often seen as more desirable candidates for higher-level positions that require advanced knowledge and the ability to manage complex projects. These positions often involve greater responsibilities, including overseeing multiple research sites, managing teams, or handling more complex aspects of clinical trials, such as data management and regulatory submissions.
Impact on Earnings:
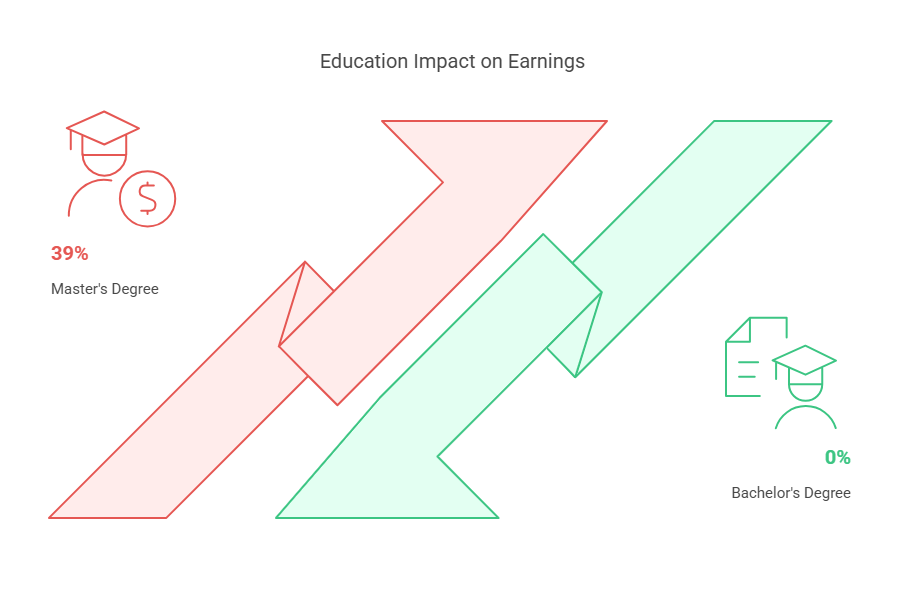
CRAs with a master's degree in Norway earn, on average, 39% more than their counterparts with only a bachelor's degree. This significant increase in earnings is reflective of the higher qualifications and the enhanced capabilities that they bring to their roles.
The increased salary compensates for the additional responsibilities and the higher level of expertise required to perform more complex tasks effectively. Employers are willing to pay a premium for the added value that master’s degree holders contribute, including improved problem-solving skills, advanced analytical capabilities, and a deeper understanding of clinical protocols and regulations.
Career Progression:
Education directly impacts career progression opportunities for CRAs. Those with higher educational qualifications are more likely to advance quickly into roles that require greater independence and leadership, such as senior CRA, clinical project manager, or even director of clinical operations.
Further, continuous education and training are valued in the clinical research field, with many professionals pursuing additional certifications or even doctoral degrees to specialize further and increase their career and salary prospects.
Navigating the Demand for CRAs in Norway
In 2025, Norway is experiencing a significant increase in the demand for Clinical Research Associates (CRAs), driven by several key factors within the country's healthcare and pharmaceutical landscapes. This boom in demand is an indicator of the vibrant opportunities available for CRAs. Here’s an in-depth look at why this is happening and what it means for professionals in the field:
Robust Healthcare System:
Norway boasts a well-established and highly efficient healthcare system that is supported by substantial public funding. This system emphasizes the importance of innovative healthcare solutions and the continual improvement of patient care practices. As a result, there is a steady demand for clinical research to test and validate the efficacy and safety of new treatments and medical interventions.
The strong infrastructure and governmental support facilitate extensive clinical studies, making Norway an attractive location for both domestic and international clinical research projects.
Thriving Pharmaceutical Sector:
The pharmaceutical industry in Norway is both dynamic and forward-thinking, with substantial investments in research and development. This sector is keen on developing new drugs and medical technologies that can address unmet medical needs or improve existing treatments.
CRAs play a crucial role in this process by ensuring that all phases of clinical trials are conducted according to strict regulatory standards and ethical guidelines. Their work helps to bring innovative products to the market safely and efficiently.
Groundbreaking Medical Devices and Pharmaceuticals:
Norway is home to a range of companies specializing in cutting-edge medical devices and pharmaceuticals. These companies are often at the forefront of developing new technologies and therapies that can transform patient care.
For CRAs, this means opportunities to work on pioneering projects involving next-generation devices and novel therapeutic drugs. The role requires a high level of expertise to manage the complexities of such trials, ensuring they deliver reliable results that can lead to regulatory approvals and ultimately, market access.
Career Opportunities and Specialization:
The increasing demand for CRAs creates numerous career opportunities, not only in conducting and overseeing trials but also in specialized areas such as regulatory affairs, data management, and patient recruitment.
Professionals can choose to specialize in particular types of clinical trials, such as those focusing on certain diseases (oncology, cardiovascular, etc.), or in trials for specific types of treatments (biologics, medical devices).
Making a Mark in Clinical Research:
The ability to contribute to significant medical advancements is a compelling aspect of working as a CRA in Norway. Professionals in this field can see the direct impact of their work on improving health outcomes and advancing medical knowledge.
Moreover, working in this environment offers CRAs the chance to develop a network of professional contacts that can enhance their careers, providing pathways to roles in clinical research organizations, pharmaceutical companies, and academic research institutions.
10 Less Commonly Known Facts About Clinical Research in Norway:
Norway invests significantly in R&D, though the exact percentage is around 2.15% of GDP in 2019, not over 5% as stated. However, this investment supports a robust clinical research environment. (Source)
Norwegian clinical trials are indeed known for their high ethical standards and rigorous safety protocols, contributing to their international reputation (Source)
Norway has one of the fastest approval times for clinical trial applications in Europe.
The Norwegian government supports clinical research through various initiatives and investments, such as funding for life science infrastructure. (Source)
Many clinical research professionals in Norway are involved in international studies, giving them unique global exposure.
The prevalence of digital health records in Norway significantly streamlines the clinical research process.
Norway’s population has one of the highest rates of participation in clinical trials per capita in the world.
Norwegian research facilities are among the most modern in the world, equipped with state-of-the-art technologies.
There is a growing trend in Norway towards the use of artificial intelligence in clinical research.
Norway has specific grants available for young researchers starting their careers in clinical research.
Explore Courses for Clinical Research Careers in Norway
Courses Available:
Conclusion
As we wrap up this expedition through the landscape of clinical research in Norway, remember that the journey doesn't end here. If you're ready to take your career to new heights, consider pursuing a certification from CCRPS. With the right training and a spirit of discovery, you could be leading the next big breakthrough in clinical research.
Frequently Asked Questions (FAQs)
-
Starting salaries for new CRAs in Norway typically begin at 357,000 NOK annually.
-
Advancing your career can be achieved through gaining experience, pursuing higher education, and obtaining specialized certifications like those offered by CCRPS.
-
A bachelor's degree in life sciences is typically required, with a master's degree providing a significant advantage.
-
Yes, the demand for CRAs in Norway is growing, driven by expansive research initiatives and regulatory needs.
-
Apart from CRAs, positions like Clinical Research Coordinators and Project Managers are also highly sought after.
-
Certifications such as ICH-GCP and Advanced Clinical Research Project Manager are crucial for career enhancement.
-
Certified professionals often earn significantly more, reflecting their advanced skills and qualifications.
-
Norway offers a robust healthcare system, competitive salaries, and opportunities to work on cutting-edge research.
Clinical research is a field with immense career potential worldwide. Clinical Research Associates (CRAs) are among the most sought-after professionals in this field. CRAs monitor labs and research processes, making them crucial for the development of new therapies. This position offers financial stability and abundant travel opportunities. In this guide, we’ll explore the prospects of becoming a CRA in Norway and the average salary in Norway for clinical research roles in 2025.
Clinical Research Associate (CRA) Salary
As of 2024, the average monthly salary for a Clinical Research Associate (CRA) in Norway is 684,000 NOK. Depending on your experience and the region you are based in, your earnings can range from 314,000 NOK per month to 1,090,000 NOK per month.
Experience-Based Salary Trends:
Less Than 2 Years of Experience: CRAs with under two years of experience earn an average of 357,000 NOK per year.
2-5 Years of Experience: After gaining 2-5 years of experience, CRAs see a 34% salary increase, with an average annual salary of 477,000 NOK.
5-10 Years of Experience: With 5-10 years of experience, the salary jumps significantly by 48%, bringing the average to 705,000 NOK per year.
For professionals interested in boosting their skills and qualifications, the CRA course is an excellent option to enhance your career prospects.
Education and Earning Power
To work as a CRA in Norway, a bachelor’s degree is the minimum educational requirement. However, master’s degree holders enjoy an advantage in the job market. CRAs with a master's degree earn, on average, 39% more than those with just a bachelor's degree.
For those seeking to further their education and advance their careers, courses such as the Clinical Research Coordinator and Pharmacovigilance Certification can help expand your knowledge and elevate your earning potential.
With the growing demand for highly skilled professionals in clinical research, Norway presents a wealth of opportunities for career growth and development.
What is the Demand for CRAs in Norway?
In 2025, the demand for clinical research associates in Norway is expected to grow significantly. As global regulations increase and pharmaceutical companies continue to advance their research efforts, clinical research careers in Norway remain in high demand. CRAs working in Norway may find themselves working with pharmaceutical, medical device, or in-vitro diagnostic device companies, as well as contract research organizations (CROs).
If you're interested in learning more about how to become a CRA, check out our Full Guide on Becoming A CRA in 2025.
Clinical Research Roles and Salary Expectations in Norway
Norway offers a dynamic and rewarding environment for clinical research professionals. Aside from CRAs, other key positions such as Clinical Research Coordinators and Clinical Research Project Managers also have a significant presence in the country. These roles, similar to CRAs, offer competitive salaries, career growth, and opportunities to contribute to medical advancements.
For those aspiring to become part of Norway's clinical research industry, it’s essential to understand the clinical trial project management process and how it integrates into the broader clinical research framework. For additional information on project management careers, visit our Questions For a Clinical Research Project Manager.
Training Programs to Enhance Your Clinical Research Career
To succeed in the clinical research field, relevant training is essential. Programs like the Advanced Clinical Research Project Manager Certification, Good Clinical Practice (ICH-GCP), and the Clinical Trials Assistant Training are valuable for anyone looking to advance their knowledge and skills.
Conclusion
Clinical research in Norway offers exciting opportunities for professionals eager to advance their careers in a growing field. Whether you're interested in the role of a Clinical Research Associate, Clinical Research Coordinator, or Clinical Research Project Manager, Norway's clinical research industry presents a wealth of prospects for professionals with the right qualifications.
If you're looking to enhance your career with specialized training and certifications, consider enrolling in courses such as the Advanced Clinical Research Project Manager Certification or Pharmacovigilance Certification to improve your qualifications.
For more detailed guidance on becoming a CRA or to explore other clinical research roles, visit CCRPS.