The Ultimate Guide to Becoming a Clinical Research Associate (CRA) in Canada: Everything You Need to Know in 2025
Clinical Research Associate - For Canada
If you're considering a career in healthcare or clinical research, becoming a Clinical Research Associate (CRA) presents a thrilling opportunity. CRAs enjoy a dynamic work setting and lucrative career prospects in this rapidly expanding field. This ultimate guide is designed to provide comprehensive insights into the CRA career, applicable whether you're in Canada or aiming for global opportunities. Discover how enrolling in the Certified Clinical Research Professionals Society (CCRPS) course can accelerate your path to success.
By the end of this guide, you’ll have the tools, strategies, and resources to master the CRA path while maximizing your career potential.
What Is a Clinical Research Associate (CRA): The Role Explained
A Clinical Research Associate (CRA) plays a pivotal role in clinical trials, tasked with ensuring that these studies adhere to rigorous ethical, scientific, and regulatory standards. Often described as the "eyes and ears" of clinical trials, CRAs oversee site operations, ensuring data accuracy and regulatory compliance. Their critical monitoring duties are essential for the integrity and success of clinical research.
Key Responsibilities:
Monitoring trial sites for protocol adherence and data accuracy.
Ensuring that the trial complies with Good Clinical Practice (GCP) guidelines.
Validating collected data and resolving discrepancies.
Acting as a liaison between trial sponsors and site investigators.
Training site staff on compliance and reporting procedures.
CRAs travel frequently—or work remotely in the case of decentralized trials—making it a dynamic career choice for those who enjoy a mix of structure and flexibility.
Clinical Research Associate Job Description and Skills
A Clinical Research Associate (CRA) plays a crucial role in the clinical research process, ensuring that clinical trials are conducted in accordance with good clinical practice guidelines and regulatory requirements. The primary responsibilities of a CRA include:
Monitoring clinical trials to ensure compliance with the clinical trial protocol, good clinical practice guidelines, and regulatory requirements.
Conducting site visits to assess the quality of data and ensure that the clinical trial is being conducted in accordance with the protocol.
Reviewing and verifying case report forms (CRFs) to ensure that data is accurate and complete.
Identifying and addressing issues that may impact the quality of the clinical trial.
Collaborating with clinical research coordinators, investigators, and other stakeholders to ensure that the clinical trial is conducted efficiently and effectively.
To be successful in this role, a CRA must possess a range of skills, including:
Strong knowledge of good clinical practice guidelines and regulatory requirements: Understanding these guidelines is essential for ensuring that clinical trials are conducted ethically and effectively.
Excellent communication and interpersonal skills: CRAs must be able to communicate effectively with site staff, investigators, and sponsors.
Ability to work independently and as part of a team: CRAs often work autonomously but must also collaborate with various stakeholders.
Strong organizational and time management skills: Managing multiple tasks and responsibilities efficiently is crucial.
Ability to analyze data and identify trends and issues: CRAs need to be detail-oriented and capable of spotting discrepancies in clinical trial data.
Strong attention to detail and ability to maintain accurate records: Precision is key in ensuring the integrity of clinical trial data.
Ability to work in a fast-paced environment and prioritize multiple tasks: The dynamic nature of clinical trials requires adaptability and effective prioritization.
In addition to these skills, a CRA must also have a strong understanding of clinical research principles, including the design and conduct of clinical trials, data management, and statistical analysis.
Career Pathway
Entry-Level Roles such as Clinical Research Coordinator (CRC) or Clinical Trial Assistant (CTA). Clinical researchers play a crucial role in training and mentoring entry-level CRAs.
CRA I/II Levels focus on independent monitoring and cross-site management.
Senior CRA or Lead CRA positions involve mentoring or managing multiple trial teams.
Career growth can also include moving into Clinical Project Manager roles, where salaries often exceed six figures.
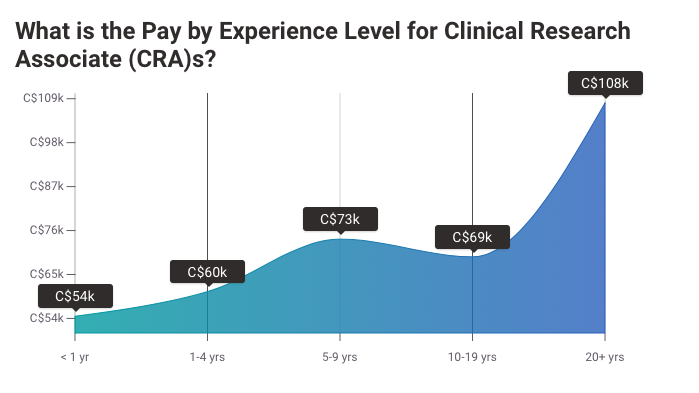
Salary Expectations: Entry-level CRAs in Canada earn approximately CAD $55,000 to $75,000, with senior roles reaching CAD $120,000 or more.
Educational Requirements to Become a CRA
Minimum Academic Credentials
Most CRA roles require a bachelor's degree in a life science field such as Biology, Biochemistry, Pharmacy, or Nursing. While an advanced degree, like a Master’s in Clinical Research, can offer a competitive edge, it is not mandatory to begin a career as a CRA.
For non-traditional candidates, certification programs like CCRPS (Certified Clinical Research Professionals Society) help bridge knowledge gaps and prepare individuals for entry-level CRA roles, even without prior research experience. These programs provide essential training to ensure compliance with the strict standards of clinical research.
Pro Tip: Canadian universities like McMaster University or York University offer specific programs in clinical trial management, blending academic learning with hands-on training.
Step Up With Certifications
Certifications enable faster entry into the CRA field and elevate your marketability. The CCRPS course is particularly advantageous as it offers a thorough curriculum across 165 modules, mentorship support, and job placement resources.
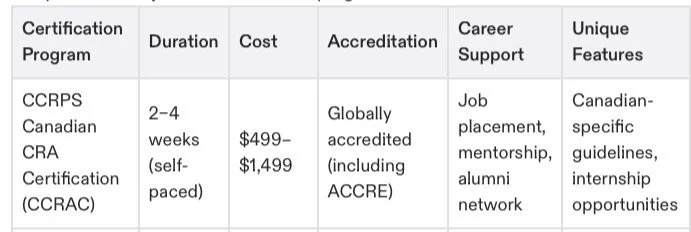
Certification Program
To determine which program suits your goals and experience, here’s a quick comparison of major CRA certification programs:
Note: CCRPS’s flexibility and comprehensive curriculum make it an excellent choice for both beginners and those transitioning from other fields.
Why the CCRPS Course Is a Game-Changer for Your Clinical Research Associate Career?
The Certified Clinical Research Professionals Society (CCRPS) provides one of the most comprehensive CRA training programs aimed at both beginners and experienced professionals.
Here’s why it's the go-to certification for boosting CRA prospects in Canada or internationally:
Globally recognized certification suitable for beginners and advanced professionals.
Offers flexible payment plans and a 100% money-back guarantee.
Includes advanced courses covering specific therapeutic areas, such as pediatric and oncology trials.
CCRPS also provides mentorship opportunities and a career-building alumni network. These exclusive benefits ensure graduates are better positioned to land higher-paying roles.
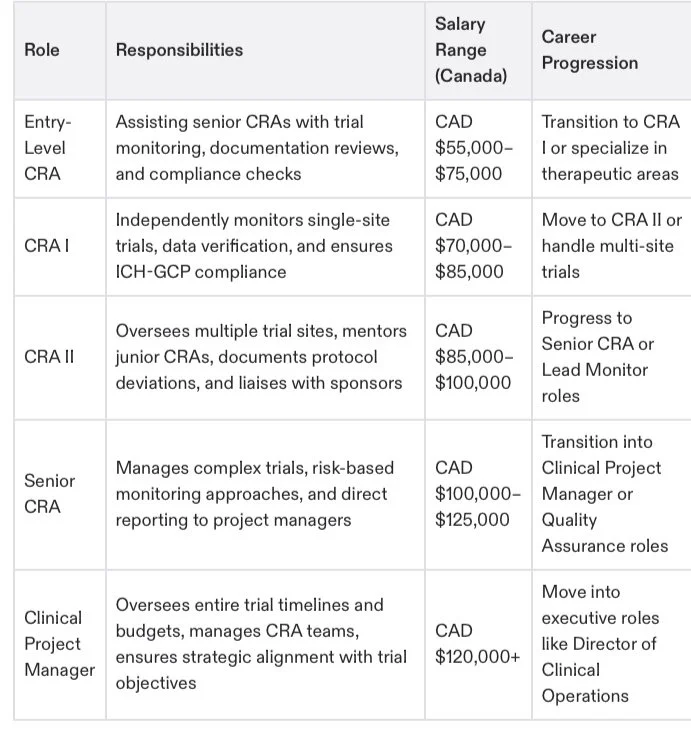
CRA Career Levels and Responsibilities
Here’s a detailed comparison of CRA roles at various career levels to help you understand your progression in this field. Many CRAs find employment opportunities within contract research organizations (CROs), which conduct clinical trials and provide essential resources for pharmaceutical firms:
Note: Specialized certifications like CCRPS can expedite progression to higher roles by equipping CRAs with advanced, niche monitoring capabilities.
Mastering Advanced Monitoring Techniques in the Clinical Research Process
The CCRPS course gives CRAs a competitive edge by offering specialized modules in risk-based monitoring, patient retention, and regulatory compliance for complex studies. Graduates often report accelerated promotions and salary growth due to these advanced skills.
Tools to Master Through CCRPS Training for Clinical Trials:
Electronic Data Capture (EDC) systems such as Medidata or Oracle Clinical.
Trial Master File (TMF) processes for streamlined documentation.
Risk-Based Monitoring Platforms adopted by leading CROs.
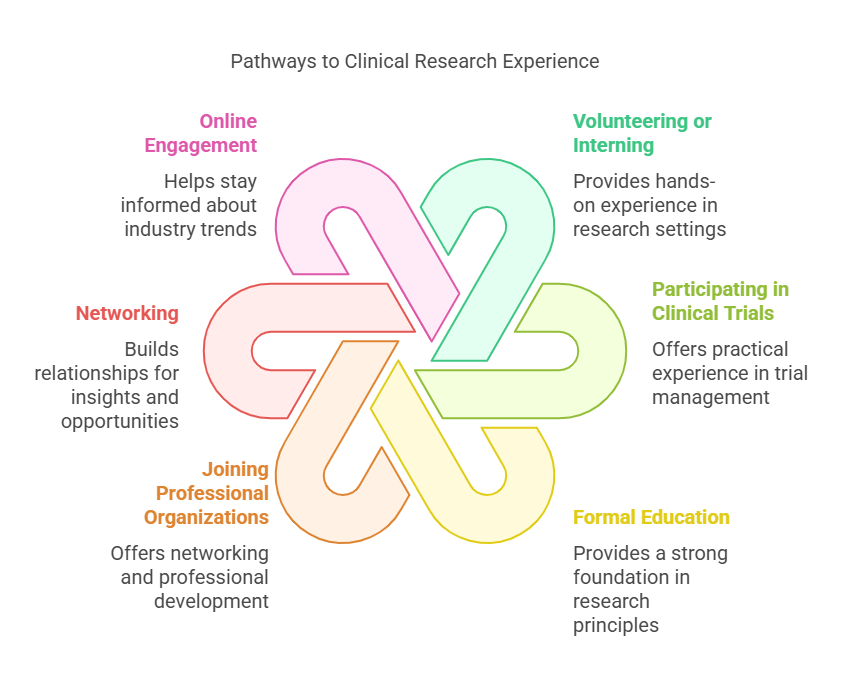
How to Get Experience in Clinical Research?
Gaining experience in clinical research is a critical step towards becoming a successful Clinical Research Associate. Here are several ways to build your experience:
Volunteering or interning at a research institution or hospital: This provides hands-on experience and exposure to the clinical research environment.
Participating in clinical trials as a research coordinator or assistant: These roles offer practical experience in managing and monitoring clinical trials.
Taking courses or earning a degree in a field related to clinical research, such as life sciences or public health: Formal education provides a strong foundation in the principles of clinical research.
Joining professional organizations, such as the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SOCRA): These organizations offer networking opportunities, resources, and professional development.
Networking with experienced clinical research professionals and seeking their advice and guidance: Building relationships with industry professionals can provide valuable insights and career opportunities.
Participating in online forums and discussion groups related to clinical research: Engaging in these communities can help you stay informed about industry trends and best practices.
It’s also important to note that many clinical research positions require a degree in a field related to life sciences or public health, as well as relevant experience. However, there are many entry-level positions available for those who are new to the field, providing a pathway to gain the necessary experience and advance in your career.
Understanding ICH-GCP Guidelines
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP) guidelines are a set of principles and guidelines that ensure clinical trials are conducted in a way that is safe, efficient, and effective. These guidelines cover a range of topics, including:
The design and conduct of clinical trials: Ensuring that trials are scientifically sound and ethically conducted.
The protection of human subjects: Safeguarding the rights, safety, and well-being of trial participants.
The collection and management of data: Ensuring data integrity and accuracy throughout the clinical trial process.
The monitoring and auditing of clinical trials: Regular oversight to ensure compliance with the trial protocol and regulatory requirements.
The reporting of adverse events and other safety issues: Prompt and accurate reporting of any issues that may affect participant safety.
The ICH-GCP guidelines are widely accepted and used by regulatory agencies around the world to ensure that clinical trials are conducted in accordance with good clinical practice. Clinical research professionals must have a strong understanding of these guidelines and be able to apply them in their work.
In addition to the ICH-GCP guidelines, clinical research professionals must also be familiar with other regulatory requirements, such as those related to electronic data capture and the use of technology in clinical research. Understanding these guidelines and requirements is essential for ensuring the integrity and success of clinical trials.
What Does a Clinical Research Associate (CRA) Do?—A Complete Role Breakdown
A Clinical Research Associate acts as the key guardian of clinical trials, ensuring compliance with regulatory and ethical frameworks. Functioning as the "eyes and ears" of trial sponsors, CRAs validate that trials operate according to Good Clinical Practice (GCP) guidelines while delivering reliable data aligned with global safety standards.
Core CRA Responsibilities
Monitoring Trial Sites: Ensure trial teams follow trial protocols and safeguard participant safety.
Data Verification: Match source documents with entries in electronic data systems like CTMS or Medidata.
Regulatory Adherence: Confirm that sites meet International Council for Harmonisation (ICH) GCP standards.
Site Training: Prepare research teams through hands-on training to maintain compliance.
Problem Resolution: Resolve deviations, errors, or logistical challenges found within trial processes.
Emerging Trends in CRA Roles for 2025
Decentralized and Hybrid Trials leveraging remote monitoring platforms like eTMF and tech-driven analytics.
Specialized CRAs focusing on therapeutic niches like oncology, gene therapy, or pediatric trials.
The rise of Risk-Based Monitoring (RBM) emphasizing targeted tracking of high-priority sites and reducing redundant oversight.
Pro Tip: Build expertise with tools like Oracle Clinical, Medidata RAVE, and Veeva Vault to accelerate entry.
Did You Know? CRAs are well-compensated for their critical roles. Salaries for entry-level CRAs begin at USD $55,000 (CAD $70,000 in Canada), and experienced professionals earn upwards of USD $120,000.
How to Become a Clinical Research Associate—Your Career Blueprint
Succeeding in clinical research doesn’t happen overnight. Follow these structured steps to create a strong foundation for this exciting role.
Obtain a Relevant Degree or Equivalent Experience
To qualify as a CRA, most hiring managers prioritize candidates with at least a bachelor’s degree in these fields:
Life Sciences (Biology, Biochemistry, Genetics)
Medicine or Nursing
Pharmacy or Biotechnology
Unlike some rigid healthcare careers, prospective CRAs can also transition from roles like clinical trial assistants or healthcare data analysts.
Considerations for Canadian CRAs
Canadian CRAs should familiarize themselves with Health Canada’s regulatory guidelines in addition to global compliance systems like FDA requirements. Institutions like McMaster University offer specific clinical trial management courses tailored for Canadians.
Build Initial Exposure Through Entry-Level Roles
Before securing a CRA role, gaining hands-on experience significantly enhances applications. Pursue:
Clinical Research Coordinator (CRC) roles handling day-to-day site management.
Administrative positions in medical research hubs like Toronto General Hospital or CRA internship programs.
Leverage volunteer programs with hours-based work to understand real-world settings.
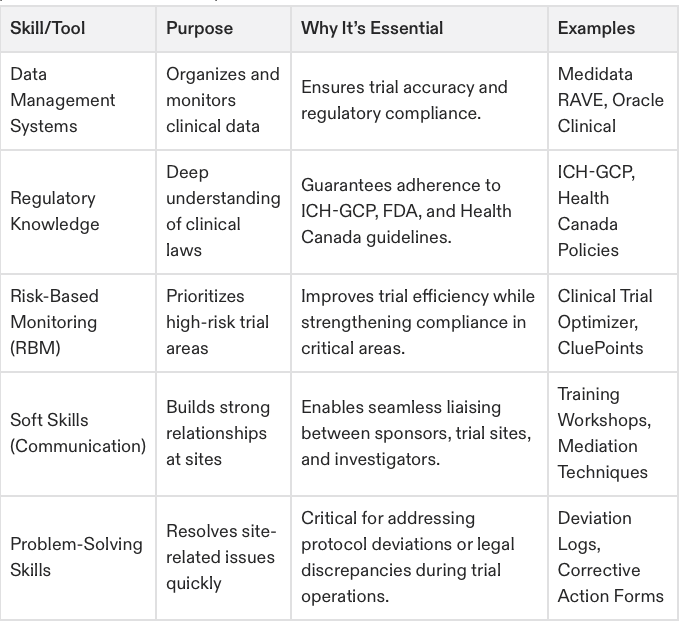
Key Skills and Tools for a CRA
This table provides a detailed comparison of the key skills and tools CRAs need to perform their role effectively.
Regulatory Compliance Excellence
Regulatory compliance is the backbone of a successful CRA career. CRAs must ensure that every aspect of a clinical trial aligns with ethical and legal standards.
Core Compliance Concepts
ICH-GCP Guidelines: A universal standard for clinical trials, ensuring participant safety and scientifically sound results.
Health Canada Regulations: Canadian CRAs must follow federal policies concerning trials conducted on Canadian soil.
FDA Compliance (for U.S.-run trials): Required if working on global or U.S.-based projects.
Pro Tip: Regularly review and stay updated on changing regulations. CCRPS modules include exclusive content on international and Canadian-specific compliance.
Effective Communication Strategies for CRAs
For CRAs, communication is not optional—it’s a decisive factor in trial success. Whether training site staff, interacting with sponsors, or resolving issues, mastering communication ensures clarity.
How to Communicate Effectively as a CRA?
Be Proactive: Schedule regular check-ins with study sites to preempt potential protocol deviations.
Tailor Messages: Adapt tone and detail based on your audience, whether site managers, sponsors, or healthcare professionals.
Use Best Practices in Documentation: Every CRA should prioritize error-free, comprehensive communication logs for trial traceability.
Tool to Try: Try role-based simulations through the CCRPS Fellowship Program to sharpen both verbal and electronic communication.
Career Advancement Tips for CRAs
Whether you’re just starting or seeking to climb the career ladder, follow these tips to accelerate your progress in clinical research.
Get Certified: Ensure your resume reflects globally recognized credentials like CCRPS certification and the CRAC credentialing exam (Canada).
Specialize in Therapeutic Areas: Advanced certifications in oncology or cardiovascular trials improve your standing within niche fields.
Broaden Networking Opportunities: Join CRA-specific organizations, such as the Canadian Clinical Trials Network, or attend industry events to discover hidden job opportunities.
Key Points on Excelling as a Clinical Research Associate
Mastering the CRA role requires more than technical knowledge—you need strategic actions, strong interpersonal skills, and certification. By following this guide’s recommendations, CRA candidates worldwide, particularly in Canada, can confidently pursue fulfilling and lucrative careers.
From enhanced certifications like CCRAC to advanced tools like RBM tracking systems, being innovative in your career opens avenues for accelerated growth. Start where you are, get certified, and watch your position in the clinical research world evolve into a leading role.
Get started today on your Canadian CRA career!
Enroll in CCRPS to Begin Your Journey
Payscale Canadian CRA Salary
Payscale Canadian Pay Difference CRA
Clinical Research Certification Specifically for Canadian CRAs
CCRPS offers one of the only online certifications specifically designed for entry-level and mid-level professionals in clinical research associate knowledge. We prepare you to understand and apply all skills required to secure a job in clinical research.
FAQS
How much does a CRA make a year?
A Clinical Research Associate (CRA) typically earns an annual salary ranging from $60,000 to $100,000, depending on experience, location, and the company they work for. Senior CRAs and those with specialized skills may earn higher salaries.
How do you qualify for CRA?
To qualify as a CRA, candidates usually need a bachelor’s degree in life sciences, nursing, or a related field. Additionally, experience in clinical research, such as working in clinical trials or in a healthcare setting, is often required. Some positions may also require specific certifications like the Certified Clinical Research Professional (CCRP).
How do I become an independent CRA?
To become an independent CRA, you need substantial experience in the field of clinical research, usually at least 2-5 years. Building a network of industry contacts, gaining a strong understanding of compliance and regulatory requirements, and possibly obtaining advanced certifications will help in establishing yourself as a freelance or contract CRA.
Can you be a CRA without a degree?
While most CRA positions require a bachelor’s degree, it is occasionally possible to work in clinical research without one, particularly if you have extensive experience in a related field, such as nursing or medical technology, or have worked your way up through clinical trial support roles.
How many levels of CRA are there?
There are typically three levels of CRAs: Entry-Level CRA, CRA II, and Senior CRA. Progression through these levels depends on experience, expertise, and the successful oversight of clinical trials. Each level entails greater responsibilities and usually requires additional training and experience.
Want to Learn More About Clinical Research?
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course