How To Become a Research Assistant in UAE (Dubai): Everything You Need to Know in 2025–2026
In the UAE’s fast-growing biomedical and clinical trial sector—especially in Dubai—Research Assistants (RAs) aren’t just data gatherers. They’re the operational core of principal investigator teams, trusted to support ethics submissions, maintain source documentation, process lab samples, and assist in adverse event tracking. With the UAE's healthcare and academic research investments expected to cross AED 4 billion by 2026, certified RAs are now among the most in-demand entry-to-mid-level hires across hospitals, CROs, and sponsor-backed trial sites.
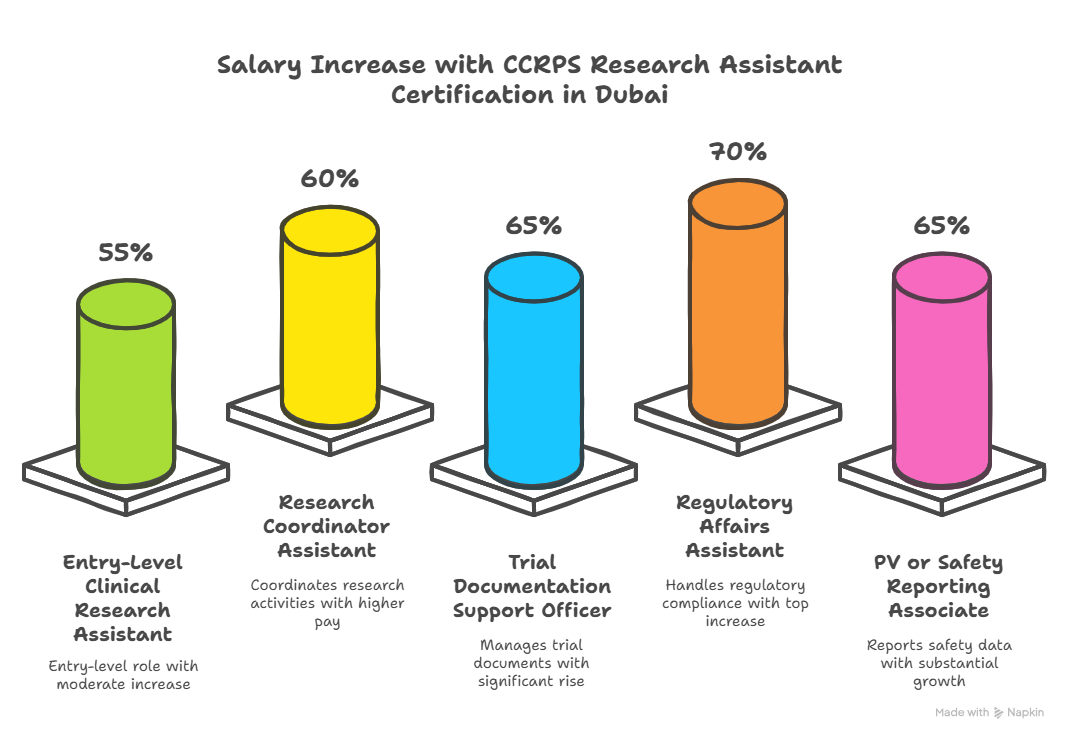
More importantly, the right Research Assistant certification doesn’t just qualify you to work—it directly impacts your earning power. Between 2023 and 2025, Dubai-based RAs who completed advanced certification programs reported salary jumps of 32% to 51%, particularly those who transitioned from admin, pharmacy, or nursing roles into formal GCP-aligned RA jobs. This guide walks you through how to become one, what skills you'll need, and the certifications that matter most to sponsors, academic research centers, and regulatory-compliant private clinics in the UAE.
What Is Research Assistant Certification in UAE (Dubai) Exactly? Skills Required and Jobs Explained
In Dubai’s competitive clinical research job market, a Research Assistant (RA) certification serves as more than just a credential—it’s formal proof that you can operate within MOHAP, DHA, and ICH-GCP standards. Certified RAs are trusted to assist with site startup, data collection, trial documentation, protocol compliance, and patient follow-ups in clinical trials. Whether supporting Phase I hospital-based studies or decentralized trials funded by international sponsors, employers across UAE trial networks now expect RAs to be compliance-trained, audit-ready, and documentation-proficient.
What Skills Does a Certified Research Assistant in UAE Actually Gain?
Manage data entry, complete case report forms (CRFs), and maintain e-source records
Support clinical protocol implementation, patient screening logs, and informed consent workflows
Maintain regulatory binders like ISF, TMF, and logs required under MOHAP, DHA, and ICH-GCP standards
Document and escalate serious adverse events (SAEs) and adverse events (AEs)
Coordinate lab kits, courier pickups, patient visits, and site logistics
Prepare trial sites for audits, monitoring visits, and regulatory inspections
What Jobs Can You Apply for With Research Assistant Certification in Dubai?
Clinical Research Assistant (CRA) – at CROs, hospitals, and sponsor-backed trial sites
Research Coordinator or Trial Support Officer – site-based or decentralized
Documentation & Compliance Officer – managing TMF, ISF, and consent form accuracy
Pharmacovigilance Assistant – supporting safety documentation and AE case tracking
Clinical Trial Operations Assistant – managing site logistics and patient coordination
Regulatory Affairs Support Staff – assisting in MHRA, MOHAP, and sponsor-facing audits
Why Should You Get Research Assistant Certification to Work in UAE (Dubai)?
Without formal certification, most jobseekers in Dubai are filtered out of research roles during the first round of hiring—especially when applying to CROs, sponsor-funded hospitals, and academic trial centers. Even with a background in nursing, pharmacy, or lab tech, lack of GCP-compliant RA training is viewed as a compliance risk by sponsors and PI teams. Certified Research Assistants, on the other hand, are prioritized for roles involving site documentation, AE tracking, and regulatory readiness because they’ve proven they understand UAE trial conduct standards and can contribute from day one. It’s the fastest way to pivot into clinical research, even without prior trial experience.
| Career Factor | Without Certification | With Research Assistant Certification |
|---|---|---|
| Job Eligibility | Often excluded from sponsor-facing or CRO research roles | Eligible for CRA, RA, and regulatory support positions in UAE trials |
| Starting Salary Range | AED 4,500–6,000/month | AED 7,000–9,000/month (plus sponsor bonuses) |
| Day-One Responsibilities | Limited to administrative support tasks | Trusted with AE documentation, TMF, screening logs, site prep |
| Compliance Confidence | Requires supervision during audits or monitoring visits | Prepared for MHRA, MOHAP, and sponsor audits independently |
| Career Growth Track | Flat progression in non-clinical assistant roles | Progression to CRC, PV Officer, Regulatory Associate in 1–2 years |
Which Certification Should You Choose to Become a Research Assistant in UAE (Dubai)?
In Dubai, the wrong certification can cost you both time and job opportunities. Many programs offer generic medical admin training or outdated trial assistant overviews, but they don’t cover ICH-GCP, UAE-specific ethics submission standards (MOHAP/DHA), AE reporting, or real-world trial documentation. These gaps show up fast in CRO interviews or sponsor audits. If you're aiming for a career in clinical trials—not just admin work—you need a certification that teaches real compliance, not theory.
The CCRPS Advanced Clinical Research Assistant Certification (ACTAC) is built exactly for this. Designed for premeds, new grads, and career switchers, it requires no prior experience and delivers a structured pathway into global clinical trial roles. With 115+ interactive lessons, 112 CPD-accredited hours, and scenario-based training across GCP, AE reporting, IRB workflows, TMF/ISF prep, CRFs, CTMS, eConsent, and decentralized trials, it covers everything you need to operate confidently in CRO, hospital, or sponsor-backed environments. Graduates receive instant certification, a LinkedIn badge, and access to live mentoring sessions, job coaching, and downloadable tools. You can complete it in 2–6 weeks, entirely online and at your own pace—with lifetime access and no renewal fees. Recognized across 40+ countries, including Dubai, Abu Dhabi, and Sharjah, it prepares you to walk into interviews already knowing source documentation, audit workflows, and regulatory compliance—not just job titles.
| Feature | Typical RA Courses (Others) | CCRPS Advanced Clinical Research Assistant Certification (ACTAC) |
|---|---|---|
| Accreditation | Unaccredited or limited to regional CPD | Accredited for 112 CPD hours, globally recognized with no renewal fees |
| Curriculum Depth | Basic GCP overview and admin forms | 115 interactive lessons across GCP, AE reporting, CTMS, CRFs, and patient recruitment |
| Learning Format | Static slides, outdated PDFs, no interaction | Scenario-based, simulation-driven, with binder prep and monitoring workflows |
| Career Target | Trains for general healthcare support | Built for premeds, new grads, and switchers entering real clinical trial roles |
| Pace & Flexibility | Fixed duration, limited access | 100% online, self-paced, bootcamp optional, lifetime access included |
| Instructor & Team | Generic or celebrity-led, no access to actual instructors | Led by expert clinical trial professionals with transparent support team |
| Job Placement Support | None or generic certificate only | Includes live mentoring, resume prep, interview coaching, and downloadable tools |
| Career Outcomes | Admin staff or site assistant only | Prepares you for RA, Study Coordinator Assistant, Regulatory Affairs Assistant roles |
| Recognition & Reach | Limited to one region or sponsor | Recognized by CROs, research hospitals, universities, and global trial sponsors |
| Salary Impact | Low entry-level wages | Typical salary $25K–$70K+ depending on trial type and CRC/CRA path |
Why CCRPS Clinical Research Assistant Certification Will Be a Game Changer for Your Career in UAE (Dubai)
In the UAE, especially in Dubai’s fast-expanding clinical research sector, most entry-level hires without certification are limited to observer or admin-only roles. Sponsors, CROs, and PI teams increasingly expect source-ready, GCP-trained Research Assistants who can contribute on Day 1. That’s where the CCRPS Advanced Clinical Research Assistant Certification (ACTAC) makes all the difference. It equips candidates with real documentation skills, not just theoretical knowledge—preparing them to manage AE/SAE logs, protocol screening, binder audits, and site visit support across Phase I–IV studies.
Between 2023 and 2025, certified professionals in Dubai reported salary increases ranging from AED 2,000 to AED 5,500/month within 6–12 months—especially those who moved from pharmacy, nursing, or lab backgrounds into fully sponsor-facing RA roles. This upward shift wasn’t based on tenure—it was directly tied to certification, document-handling capability, and audit-readiness.
Summarizing All You Need to Know About Getting Your Research Assistant Certification in UAE (Dubai)
If you’re serious about stepping into the UAE’s booming clinical trials industry—especially in Dubai’s sponsor-backed, audit-intensive research environment—then certification isn’t optional, it’s strategic. The CCRPS Advanced Clinical Research Assistant Certification (ACTAC) gives you more than a credential; it gives you operational readiness, documentation fluency, and job-winning differentiation. Whether you’re a premed, nurse, pharmacist, or a fresh graduate, this is your gateway to real trial site integration, regulatory support, and sponsor trust.
| Category | Details |
|---|---|
| Certification Name | CCRPS Advanced Clinical Research Assistant Certification (ACTAC) |
| Ideal Candidates | Premeds, nurses, pharmacists, lab techs, and career switchers (no prior experience required) |
| Accreditation | CPD-accredited, 112 hours; globally recognized with no renewal required |
| Curriculum Format | 100% online, self-paced; 115 lessons + simulations, scenario-based cases, live mentoring |
| Key Training Topics | AE/SAE, CTMS, CRFs, eConsent, TMF/ISF, adaptive trials, rare-disease protocols |
| Certification Exam | 50-question proctored exam, 2 attempts included, PDF certificate + LinkedIn badge |
| Placement Support | Resume coaching, job-matching, live mentoring, and 40+ country support system |
| Expected Salary (Dubai) | AED 7,000–12,000/month depending on placement, site type, and role advancement |
| Career Outcomes | RA, Study Coordinator Assistant, Regulatory Affairs Assistant, PV Officer |
Frequently Asked Questions
-
Yes. The CCRPS Advanced Clinical Research Assistant Certification (ACTAC) is CPD-accredited and globally recognized, including in the UAE. It aligns with MOHAP, DHA, and ICH-GCP standards, making it acceptable for positions in Dubai’s hospitals, CROs, and sponsor-backed clinical trials. Graduates have used it to qualify for RA and documentation roles at trial sites involved in Phase I–IV studies, often bypassing the typical 6–12 month waiting period for on-site eligibility. Hiring teams value its focus on TMF, AE reporting, and regulatory prep—skills that match real job responsibilities.
-
The course is 100% online and self-paced, with most learners finishing in 2–6 weeks. You get lifetime access, so you can return to it as needed. It includes 115 interactive lessons, a 50-question proctored final exam, and access to weekly live mentoring sessions. There’s no expiration or renewal fee. Once you pass the exam (70% required, two attempts included), you’ll receive a PDF certificate and LinkedIn badge for use in job applications or sponsor onboarding.
-
ACTAC prepares you for roles like Clinical Research Assistant, Study Coordinator Assistant, Regulatory Affairs Associate, and Documentation Officer. In Dubai, these jobs exist at sponsor sites, CROs, hospitals, and private research centers. Many graduates enter hybrid positions that combine AE documentation, binder prep, and trial logistics coordination. The certification signals to employers that you are audit-ready and GCP-compliant—two traits that significantly speed up your hiring process into formal trial teams.
-
No. The program is designed for new grads, premeds, and healthcare professionals transitioning into research. You do not need prior experience or a science degree. Everything from clinical trial phases, GCP, eConsent, CTMS, TMF, AE/SAE, and ethics documentation is covered from the ground up. It’s especially useful for nurses, pharmacists, or lab staff in Dubai looking to enter research while leveraging their healthcare background into higher-paying, documentation-focused roles.
-
Yes. ACTAC includes access to resume reviews, job prep guides, downloadable documentation templates, and weekly live mentoring sessions focused on interview coaching, clinical site scenarios, and sponsor-readiness. Graduates also receive guidance on applying to roles across the UAE and 40+ supported countries. Many report securing interviews within 2–4 weeks of certification, especially when applying to CROs or trial centers with urgent RA staffing needs.
Sources: Economic Research Institute, CCRPS