What are Clinical Research Fast Track Programs?
Ever felt like you’ve hit the snooze button a few too many times when it comes to your career? In the fast-paced world of clinical research, waiting around for the perfect opportunity isn't always an option—so why not fast-track it?
Imagine diving straight into the clinical research field with a program that’s designed to get you certified, skilled, and ready for action in as little as two weeks. Sounds too good to be true? Well, in 2025, clinical research fast track programs are no longer just a luxury—they’re a necessity for professionals looking to jumpstart their careers and meet the industry's ever-increasing demands.
If you’re ready to stop dragging your feet and start climbing the clinical research ladder at lightning speed, then you’re in the right place! This blog is your ultimate guide to understanding Clinical Research Fast Track Programs, how they work, and why they are the secret weapon for anyone who’s serious about thriving in the world of clinical trials.
The Rise of Clinical Research Fast Track Programs
Over the ten years or so there has been a surge, in the significance of clinical studies. From medications to medical equipment the demand, for experts capable of maneuvering through intricate clinical trials has surged. However here's the tricky part— clinical research is not a one size fits all domain. The positions are specialized the rules are strict. The education required to step into this field may require years.. Guess what? Things have changed now!
Enter the Clinical Research Fast Track Program. These innovative programs have emerged as the game-changers for aspiring clinical research coordinators (CRCs), clinical research associates (CRAs), and other professionals looking to fast-track their way into this booming industry. But what exactly are they?
What is a Clinical Research Fast Track Program?
In terms a Rapid Clinical Research Training Program is an fast paced program aimed at preparing individuals, with the essential knowledge and abilities required to pursue a career in clinical research.' These programs are tailored to professionals keen on positions such as Clinical Research Coordinator (CRC) Clinical Research Associate (CRP) and Clinical Research Manager' providing a route, to obtaining certification.
The great thing, about these programs is that you can enroll instantly and earn your certification quickly! Unlike paths that may take years to complete a program you can get certified in just two weeks with a fast track program.Whether you are new to the field or considering a career change this program equips you with the skills needed to start strong, from the beginning.
Why Clinical Research Fast Track Programs are Essential in 2025
The increasing need, for experienced clinical research experts is driving the popularity of fast track programs as a component of career advancement within the field. With the growth of trials in cutting edge areas like biotechnology and gene therapy leading the way in advancements such as pharmacovigilance,the demand, for professionals capable of overseeing and conducting trials with efficiency and integrity has never been greater.
These programs offer a structured and efficient approach to training, focusing on practical skills like:
ICH GCP Compliance: Ensuring that all trials are conducted according to international ethical guidelines.
Protocol Adherence: Understanding how to follow and manage clinical trial protocols with precision.
Risk Management: Learning how to identify, assess, and mitigate risks in clinical trials.
Pharmacovigilance: Gaining expertise in monitoring and reporting adverse events during clinical trials.
In short, clinical research fast track programs not only save time but also help you gain highly valuable industry skills at an accelerated pace.
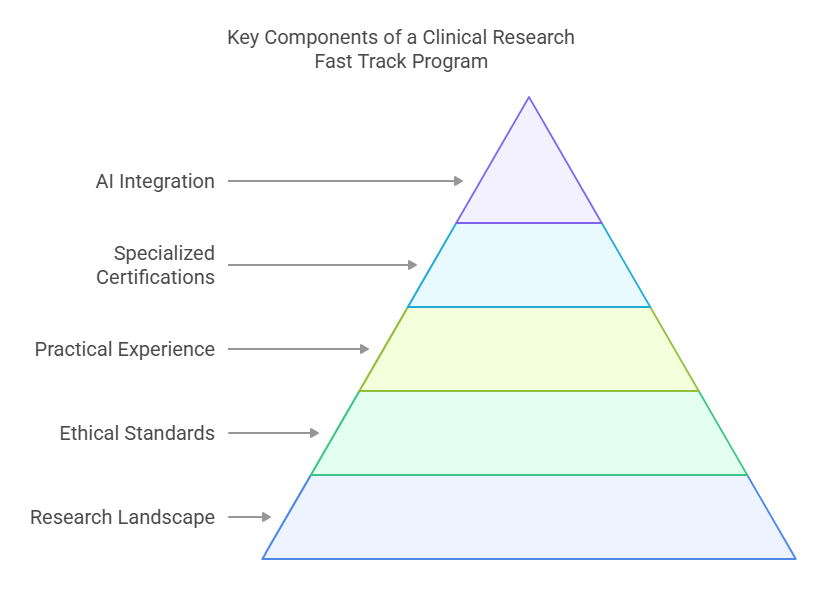
Key Components of a Clinical Research Fast Track Program
A comprehensive Clinical Research Fast Track Program covers a variety of essential topics to ensure that students are well-prepared for their roles in the clinical research field. These components typically include:
Understanding the Clinical Research Landscape
This module introduces participants to the core roles in clinical research, such as Principal Investigators (PIs), Clinical Research Coordinators (CRCs), and Clinical Research Associates (CRAs). It also covers the regulatory bodies that govern the industry, including the FDA and EMA.
Ethical Standards & Informed Consent
Compliance with Good Clinical Practice (GCP) and Institutional Review Board (IRB) standards is non-negotiable in clinical research. This part of the program delves deep into ethical research practices, emphasizing informed consent and participant rights.
Hands-On Training & Practical Experience
Perhaps the most important aspect of the program is the opportunity for hands-on training. Fast track programs often include internships or practical exposure at clinical trial sites, allowing students to work directly with clinical teams and gain real-world experience.
Specialized Certifications
Some programs offer specialized certifications in key areas of clinical research, such as Pharmacovigilance Certification or Clinical Research Management. These certifications further enhance your qualifications and set you apart in the job market.
The Role of AI and Machine Learning
In 2025, artificial intelligence and machine learning are no longer buzzwords—they’re shaping the future of clinical research. As a result, many fast track programs now integrate these technologies into their training, allowing students to better analyze clinical trial data and predict patient outcomes.
Career Opportunities After Completing a Clinical Research Fast Track Program
Completing a Clinical Research Fast Track Program opens the door to a world of exciting career opportunities. As the clinical trials industry expands, roles such as Clinical Research Coordinator (CRC), Clinical Research Associate (CRA), and Principal Investigator (PI) are in high demand.
Here’s a quick overview of the most popular career paths after completing a fast track program:
Clinical Research Coordinator (CRC): You’ll be responsible for overseeing the day-to-day operations of clinical trials, ensuring compliance with protocols, and managing participant recruitment and retention.
Clinical Research Associate (CRA): CRAs monitor clinical trials and ensure they run smoothly. You’ll inspect trial sites, review data, and collaborate with regulatory bodies.
Principal Investigator (PI): As a PI, you’ll lead clinical trials, ensuring that they adhere to all regulatory guidelines and ethical standards.
Clinical Trials Assistant (CTA): A CTA supports the clinical research team, managing documentation and ensuring that trials are progressing as planned.
These roles not only offer competitive salaries but also provide the opportunity to work at the forefront of groundbreaking medical advancements.
The Evolution of Clinical Research Fast Track Programs
Over the years, Clinical Research Fast Track Programs have undergone significant transformations to meet the dynamic needs of the healthcare industry. Initially, these programs were designed to provide a quick entry into the field for individuals seeking to become Clinical Research Coordinators (CRCs) or Clinical Research Associates (CRAs). However, as the complexity of clinical trials increased and the demand for specialized knowledge grew, these programs evolved to offer more comprehensive training.
Hybrid Learning Models
One of the most notable advancements is the adoption of hybrid learning models. These models combine online coursework with in-person practical experiences, allowing students to gain theoretical knowledge while also applying it in real-world settings. This approach not only enhances learning outcomes but also better prepares graduates for the challenges they will face in the field.
Integration of Advanced Technologies
Another significant development is the integration of advanced technologies such as Artificial Intelligence (AI) and Machine Learning (ML) into the curriculum. These technologies are increasingly being used in clinical research to analyze complex data sets, predict patient outcomes, and streamline trial processes. By incorporating these tools into training programs, aspiring professionals are better equipped to navigate the evolving landscape of clinical research.
Global Standardization
As clinical trials become more global, the need for standardized education and training programs has become more pressing. Fast track programs are adapting to these global needs by aligning with international guidelines and practices, ensuring that graduates are prepared to work in diverse and multicultural environments.
The Importance of Clinical Research Fast Track Programs
In 2025, the demand for skilled clinical research professionals has surged, with fast track programs playing a vital role in bridging the talent gap. These programs focus on:
ICH GCP Compliance and Federal Regulations: Ensuring that all trials are conducted according to international ethical guidelines.
Protocol Adherence and Deviation Management: Understanding how to follow and manage clinical trial protocols with precision.
Clinical Trial Operations and Risk Mitigation: Learning how to identify, assess, and mitigate risks in clinical trials.
Adverse Event Reporting and Pharmacovigilance: Gaining expertise in monitoring and reporting adverse events during clinical trials.
Participant Recruitment, Training, and Retention: Developing strategies to effectively recruit, train, and retain participants in clinical trials.
By enrolling in a clinical research fast track program, students gain a competitive edge by learning critical industry skills in a structured and time-efficient manner.
10 Less Commonly Known Facts About Clinical Research Fast Track Programs:
The first Clinical Research Fast Track programs were introduced in the early 2000s as a response to the increasing demand for clinical research professionals.
Many fast track programs now offer virtual internships, allowing students to gain experience without leaving their homes.
AI is not just used for data analysis but is also used to improve the design of clinical trials, predicting potential outcomes before trials even begin.
Clinical research fast track programs can be a stepping stone to leadership roles in clinical research management, with specialized tracks available for future managers.
Many fast track programs collaborate with major pharmaceutical companies, providing students access to top-tier networks.
The success rate of fast track graduates finding employment in clinical research has increased by over 30% in the last five years.
Clinical trials are projected to grow by 10% annually, increasing the demand for qualified CRCs and CRAs.
Some programs allow students to specialize in niche areas of clinical research, such as gene therapy or oncology trials.
Certification from a fast track program can often be used as credit towards more advanced degrees in clinical research.
Fast track programs are evolving to incorporate more global perspectives, with some programs offering international training opportunities.
Final Thoughts
As the demand for clinical research professionals continues to grow, enrolling in a clinical research fast track program is an excellent way to accelerate your career. These programs provide structured training, hands-on experience, and certification within weeks, making them an attractive option for aspiring clinical research professionals.
If you're looking to enroll in a fast track certification program, you can start within minutes and obtain certification in as little as two weeks. This streamlined process is crucial, as the role of a Clinical Research Coordinator is highly technical and requires a deep understanding of ICH GCP guidelines, FDA regulations, and clinical trial protocols.
Elevate your career prospects by enrolling in a clinical fast track course designed for aspiring research professionals.
For comprehensive training in clinical research, consider exploring the programs offered by CCRPS. They provide the best Good Clinical Practice Certification, Research Assistant Training Program, and Clinical Research Management Training, all designed to fast-track your career and equip you with the expertise needed to excel in the clinical research field.
Frequently Asked Questions (FAQs)
What is the duration of a Clinical Research Fast Track Program?
Fast track programs typically range from a few weeks to a couple of months, depending on the depth of the curriculum and the mode of learning. Most programs offer certification within 2-4 weeks.
Can I work while enrolled in a Clinical Research Fast Track Program?
Yes, many programs offer flexible schedules that allow students to work part-time while completing their training. Online modules are often self-paced, making it easier to balance work and study.
Do I need a science background to enroll in a Clinical Research Fast Track Program?
While having a background in life sciences or healthcare is beneficial, it’s not always required. Many programs welcome students from diverse fields and provide foundational knowledge in clinical research.
Is financial aid available for Clinical Research Fast Track Programs?
Some programs offer financial aid or payment plans to make training more accessible. Be sure to inquire about any available scholarships or funding options before enrolling.
What are the most important skills I’ll learn in a Clinical Research Fast Track Program?
Key skills include understanding clinical trial protocols, ensuring ethical compliance, managing risks, and handling adverse events, along with technical skills in data analysis and reporting.
Are Clinical Research Fast Track Programs recognized globally?
Yes, many fast track programs are accredited and recognized by global regulatory bodies, ensuring that graduates are well-prepared to work in international clinical trials.
How do I find the best Clinical Research Fast Track Program?
Look for accredited programs with comprehensive curricula, experienced instructors, and strong industry connections. Read reviews from past students and ensure the program offers practical, hands-on training.
What career prospects can I expect after completing a Clinical Research Fast Track Program?
Graduates can pursue careers as Clinical Research Coordinators, Clinical Research Associates, and other roles in the clinical research field. These positions are in high demand and offer competitive salaries.