How to Pass the CRC Exam on Your First Attempt: Proven Strategies
Getting certified as a Clinical Research Coordinator (CRC) isn’t just about passing a test—it’s about proving you're ready to lead real-world clinical trials with precision and regulatory compliance. But the CRC exam isn’t easy. It’s packed with protocol-specific questions, GCP nuances, and operational logistics that demand deep understanding, not surface-level memorization. Whether you're a recent graduate or already in the field, first-time exam success requires more than casual prep—it takes strategy, structure, and insight.
This guide lays out every high-impact step to pass the CRC exam on your first try. From understanding the structure of the test to mastering study blueprints, avoiding costly mistakes, and conditioning your mindset for test day—we’ve built this roadmap for serious candidates aiming for first-time success. And if you’re pursuing the CCRPS Clinical Research Coordinator Certification, we’ll show you exactly how this program prepares you better than any generic study method.
Understand the CRC Exam Structure
Passing the CRC exam begins with understanding its architecture. Without clarity on how the test is built, you risk wasting time on low-yield material while missing what actually matters. The Clinical Research Coordinator exam—especially through the CCRPS Clinical Research Coordinator Certification—is designed to test both theoretical knowledge and applied judgment. It mimics the complexity of real-world site coordination, subject management, and regulatory documentation. Instead of focusing solely on medical content, it emphasizes process, compliance, ethics, and decision-making under pressure.
To approach this systematically, you need to break the exam into two layers: what’s covered (domains) and how it’s delivered (duration, question style, and grading). Below, we explore both, starting with the core content areas.
Domains Covered in the Exam
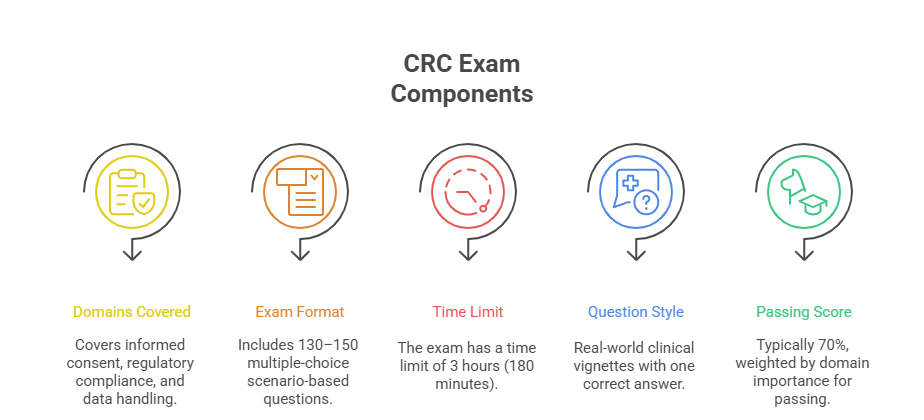
The CRC exam is domain-based, meaning questions are categorized across thematic clinical research responsibilities. While the exact blueprint varies by certifying body, CCRPS’s certification program centers on globally relevant job functions.
Key domains include:
Study Startup & Site Management
Candidates are tested on site qualification, feasibility assessments, contract/budget review, and IRB/EC preparation. A strong grasp of startup timelines and sponsor-CRO dynamics is critical.Informed Consent Process
You must understand every aspect of informed consent—patient communication, documentation timing, re-consent procedures, and legal/ethical implications under GCP and ICH guidelines.Subject Recruitment & Retention
Expect scenario-based questions on screening logs, dropout risk mitigation, and protocol inclusion/exclusion criteria interpretation.Regulatory Compliance & Documentation
From investigator site files to SAE reporting and FDA audits, this domain tests how well you can maintain a compliant trial environment.Source Data Verification (SDV) & Monitoring Prep
Questions here assess your understanding of CRF accuracy, source data review, monitor readiness, and query resolution.GCP, ICH, and FDA/EMA Guidelines
GCP violations, regulatory timelines, and ethical decision-making play a major role in the scoring algorithm. Understanding the difference between guideline types is non-negotiable.
Mastering these domains requires real scenario interpretation, not just theory. CCRPS integrates simulations and global protocol examples to ensure candidates aren’t just memorizing—they’re learning how to act.
Exam Duration and Format
The CRC exam isn’t long just for the sake of endurance—it’s long to test your mental consistency, time management, and ability to apply knowledge under cognitive fatigue. Here’s what to expect:
Total Questions: Approximately 130–150 multiple-choice items
Duration: Up to 3 hours, depending on the provider
Format: Online proctored or in-person testing center
Question Type: Scenario-based MCQs with only one correct choice
Each question typically presents a clinical situation—e.g., a protocol deviation or subject safety issue—then asks what the coordinator should do next. These are not trivia questions. They assess whether you think like a compliance-focused site coordinator, applying ICH-GCP rules and sponsor expectations simultaneously.
Scoring is based on a mix of accuracy and weighted domain performance. Some questions may carry more weight if they fall under critical compliance categories (e.g., adverse event handling). A score of 70% or higher is generally required to pass, but that cut-off can shift slightly by organization.
By fully grasping this structure, you can begin to tailor your study strategy—aligning effort with the real scoring logic of the exam.
Proven Study Methods That Actually Work
Studying for the CRC exam isn’t about how many hours you put in—it’s about where you focus and how strategically you retain. Many candidates fail not because they lack dedication, but because they study everything equally. The most successful test-takers reverse-engineer the exam blueprint and apply active recall and spaced repetition methods to ensure deep, test-ready knowledge.
By combining a blueprint-based approach with high-yield tools, you create a study system that mimics the mental conditions of the real exam—reducing overwhelm and improving long-term retention. Below are two methods that consistently lead to first-attempt CRC exam success.
Study Schedules Based on Exam Blueprint
The exam blueprint is your roadmap. It outlines the percentage of questions from each domain, allowing you to prioritize accordingly. CCRPS provides a blueprint aligned with the ICH-GCP competencies expected from global research sites.
To study effectively:
Break Down the Blueprint by Weight
If regulatory compliance makes up 30% of the exam, it deserves 30% of your time. Many students waste hours on general research knowledge instead of targeting protocol violations, audit readiness, and documentation.Use Time Blocks, Not Open-Ended Sessions
Study in 60-minute focused sessions with a defined topic (e.g., “SAE Reporting” or “IRB Submission Process”) and a clear outcome (e.g., “Score 80% on flashcard set”).Rotate Content With Spaced Repetition
Revisit difficult domains every 48–72 hours. Use a rotating cycle like:Day 1: Informed consent scenarios
Day 2: GCP quiz + adverse event flashcards
Day 3: Monitoring logs & data query samples
Weekly Practice Exams
Dedicate one day per week to simulate test conditions. This reinforces recall, boosts time management, and helps you identify weak spots early—before they cost you real points.Adapt to Your Learning Style
Are you a visual learner? Use process flowcharts. Prefer reading? Annotate real protocol examples. Auditory? Record summaries and replay them during passive hours.
A 6–8 week plan, built around this structure, gives you consistent mastery over every domain, not just shallow exposure.
High-Yield Resources (e.g., flashcards, Q-banks)
Not all study materials are created equal. Many free CRC prep sites are outdated or too generic. To pass your CRC exam confidently, focus only on targeted, exam-relevant resources.
Here are the highest-yield options that successful CCRPS candidates consistently rely on:
Flashcards with Clinical Scenarios
Skip vocabulary decks. Focus on scenario-based cards that test logic—e.g., “Subject misses 3 visits. What’s your next action?” These mimic the exam more closely than definition drills.Question Banks (Q-banks)
Use Q-banks that provide explanations for both correct and incorrect answers. CCRPS’s Q-bank includes hundreds of questions modeled after real sponsor/CRO audits, protocol violations, and documentation best practices.GCP Violation Case Studies
Read real inspection warning letters and protocol deviations. Turn them into flashcards or mock questions. This trains your brain to recognize patterns under exam pressure.Checklist-Based Review Sheets
Instead of studying paragraphs, work from review checklists:“5 things to do during site initiation”
“Key parts of an SAE report”
“What to log after consent”
CCRPS Simulated CRC Exams
These replicate actual exam difficulty. Every question is vetted by senior CRAs and site managers to match how real-life challenges translate into test scenarios.
Using these tools, your preparation becomes active, targeted, and efficient—reducing burnout and dramatically boosting retention rates.
| Strategy | Details |
|---|---|
| Blueprint-Based Scheduling | Allocate study time according to exam domain weightage. Prioritize high-impact areas like GCP, monitoring prep, and informed consent compliance. |
| Active Recall | Use scenario-based flashcards and verbal explanations to enhance long-term retention. Avoid passive rereading or note highlighting. |
| Spaced Repetition | Review challenging topics every 48–72 hours. Rotate through blueprint domains regularly to reinforce memory before degradation. |
| Mock Exam Day | Take one full-length timed test each week. Simulate exam conditions to practice endurance, pacing, and domain integration. |
| High-Yield Resources | Focus on tools like annotated protocols, deviation case studies, GCP violation letters, and CCRPS’s flashcard & Q-bank platforms. |
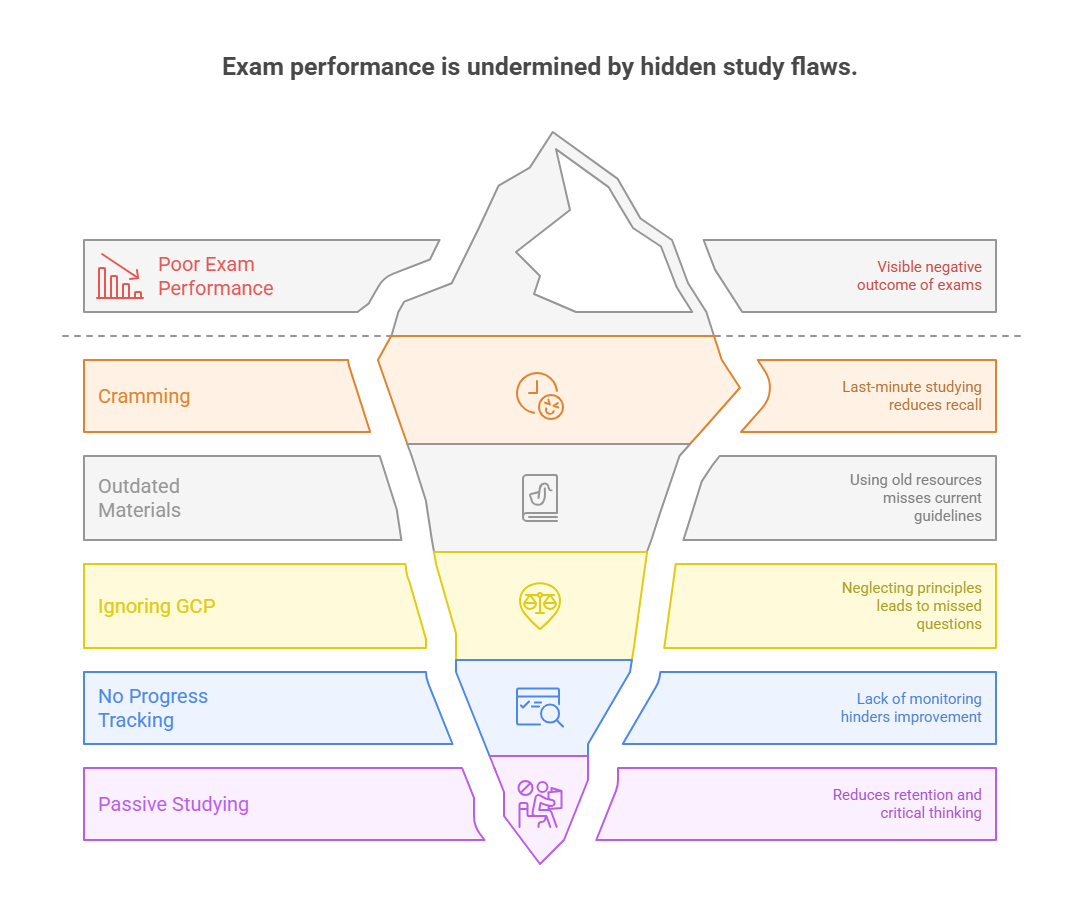
Mistakes to Avoid During Preparation
Even highly motivated candidates can fail the CRC exam if they study the wrong way. What separates first-time passers from repeat test-takers isn’t just what they know—it’s what they avoid doing. The most common prep mistakes are subtle at first but compound over weeks. They lead to knowledge gaps, test-day panic, or misaligned expectations that sabotage even the best intentions.
Below are the three most damaging mistakes that you must avoid to increase your chances of success.
Cramming Before the Test
Cramming isn’t just ineffective—it can be dangerous in the context of the CRC exam. Since the test is scenario-heavy and compliance-focused, memorization without deep understanding won’t help. In fact, it may cause confusion when two similar answers appear, and you can’t recall the logic behind the correct one.
Cramming overloads your short-term memory, leading to mental fatigue and slower recall under pressure.
The CRC exam often presents long clinical situations that require multi-step reasoning, not quick recall.
Candidates who cram tend to second-guess themselves more often, which lowers confidence and increases errors.
Instead, commit to active recall over 6–8 weeks, building up mental endurance and clarity. You’re not just learning facts—you’re learning to think like a site coordinator under protocol constraints.
Relying on Outdated CRC Material
Outdated prep resources are a silent killer. The field of clinical research evolves constantly with updates to ICH-GCP, FDA guidance, EMA protocols, and sponsor expectations. Many free resources online still teach material based on pre-2016 guidelines—or worse, skip international regulations entirely.
CCRPS’s CRC certification updates content quarterly to include real-world deviations, inspection trends, and digital protocol workflows.
Watch for red flags in outdated materials: lack of references to remote monitoring, eConsent, risk-based monitoring (RBM), or protocol deviations under COVID-era flexibility.
Relying on a static PDF or 5-year-old textbook means preparing for an exam that no longer exists.
Neglecting GCP and Regulatory Guidelines
Too many students overfocus on operational tasks (screening, visit scheduling) while ignoring the backbone of the exam: GCP compliance and regulatory ethics. Roughly 30–40% of the test evaluates how well you understand informed consent, deviation reporting, and subject rights.
Don’t skim the ICH-GCP guidelines. You need to internalize the logic, not just cite the articles.
Use mock violations and real IRB letters to simulate regulatory decision-making.
The best-prepared CRCs think like inspectors—anticipating what could go wrong, and how to fix it while staying within the rules.
Simulation & Practice Exams
No amount of passive studying can match the impact of timed, scenario-based simulation. The CRC exam tests how well you can think under pressure, filter out distractions, and choose the most compliant answer among plausible options. That’s why practice exams aren’t optional—they are essential. But not all practice tests are equal. Some build confidence. Others give you false hope. What you need are realistic, feedback-driven simulations that mirror the structure, tone, and pacing of the actual exam.
Below, we cover how to identify high-quality practice tests and use them to track your readiness with surgical precision.
What Makes a Good Practice Test
A good practice exam doesn’t just quiz you—it replicates the real challenge. It recreates the cognitive environment of the CRC exam: time-limited, decision-heavy, and ethically nuanced. Weak mock tests, on the other hand, often use trivia-style questions or unrealistic clinical scenarios that mislead you about what to expect.
Here’s what to look for:
Scenario-Based Format
Every practice question should pose a clinical or operational scenario, not just a definition. For example: “A subject misses three visits but wants to continue. What must the CRC do next?” This mimics how compliance decisions are tested on the real exam.ICH-GCP and FDA Integration
Good practice tests challenge your ability to apply regulatory frameworks in dynamic situations. You shouldn’t just recognize GCP rules—you should know how to act on them.Timed Environment
Make sure the test includes a timer. This helps condition your mental pacing and reduces exam-day stress.Detailed Explanations
Each answer—right or wrong—should come with a breakdown. Understanding why something is wrong teaches more than the correct choice alone.Adaptive Difficulty
Some of the best simulations, like those in the CCRPS CRC certification, increase in difficulty based on your performance, helping you build resilience.
Choosing the right mock test ensures you practice the exam, not just “quiz yourself.”
Tracking Progress from Mock Exams
Practice exams without performance tracking are like maps without a destination. If you’re not measuring what’s improving—and what’s not—you’re likely reinforcing your weak points without knowing it. Tracking converts practice into real progress.
Use these strategies:
Maintain a Mistake Log
After every mock test, write down the question you missed, the concept behind it, and what misled you. Over time, you’ll spot patterns—e.g., recurring mistakes in SAE reporting or site file documentation.Score by Domain, Not Just Overall
Don’t just aim for 80% overall. Track your performance in each domain: study startup, compliance, patient interaction, monitoring prep. This lets you fine-tune your focus areas.Review, Don’t Just Retake
Re-doing the same test is less helpful than reviewing why each question works. Force yourself to explain each answer as if you’re teaching it to someone else.Benchmark Weekly
Take a full-length mock every 7 days. Graph your performance to spot plateaus or regressions. If scores dip, look at sleep, focus, and revision schedule.
This method builds data-driven confidence. You’ll walk into the exam knowing where you stand—not just hoping it goes well.
| Simulation Element | Importance |
|---|---|
| Scenario-Based Questions | Replicates real CRC exam content by challenging you to apply GCP, regulatory, and protocol logic to clinical dilemmas. Ensures you’re thinking like a coordinator, not a textbook reader. |
| Timer-Enabled Practice Tests | Helps you develop a pacing strategy to complete 130–150 questions in under 3 hours. Builds mental stamina and reduces test-day time anxiety. |
| Domain-Specific Feedback | Identifies exactly which knowledge areas need more review—e.g., SAE reporting, IRB submissions, source data verification—so you don’t waste time on already-mastered content. |
| Detailed Answer Rationales | Reveals why the right choice is correct and why the others are wrong. Encourages deep reasoning instead of shallow recall, making each question a learning opportunity. |
| Performance Tracking Tools | Allows you to monitor score trends across different domains and time intervals. Helps forecast exam readiness and builds confidence through measurable improvement. |
Test-Day Confidence and Mental Conditioning
You can know everything in the CRC exam blueprint and still underperform if your mental game collapses on test day. What differentiates high scorers isn’t just content mastery—it’s cognitive clarity, time control, and emotional regulation under pressure. The CRC exam requires three hours of focus with minimal breaks, dense clinical scenarios, and ethics-based decisions that can’t be second-guessed.
That means preparing your mind and body is just as crucial as studying. Below, we break down what high-performing candidates do in the 24 hours leading up to the exam to control energy, reduce nerves, and stay sharp through every question.
Sleep, Hydration, and Breathing
Your brain can’t retrieve what it hasn’t consolidated. That’s why sleep is your most important study tool—especially the night before your exam. Pulling an all-nighter to “cram one last time” is a recipe for poor recall and slow processing.
Aim for 7–9 hours of quality sleep before the exam, especially the night prior. Avoid screens 90 minutes before bedtime to allow for deep REM cycles, where memory consolidation happens.
Stay hydrated, but don’t overdo caffeine. Dehydration impairs focus. Start hydrating the day before, and avoid energy drinks or excessive coffee that can spike anxiety mid-exam.
Use diaphragmatic breathing techniques (4–7–8 method or box breathing) in the morning and during breaks. These activate your parasympathetic nervous system, lowering cortisol and helping you stay calm and clear-headed.
Your physical state fuels your mental edge. A rested, hydrated brain processes information 20–30% faster during timed scenarios—exactly the advantage you need on high-difficulty clinical reasoning questions.
Handling Anxiety and Time Management
Even well-prepared candidates panic when faced with a tricky scenario or time crunch. What matters most is how you respond. Elite CRC candidates use structured methods to navigate nerves and pace themselves through the exam without burnout.
Use a time-allocation model:
With ~150 questions in 180 minutes, spend no more than 1.2 minutes per question. Flag and move on if unsure—don’t get stuck on one scenario.Practice “anchor mindset resets”. If anxiety spikes, stop for 5 seconds. Breathe. Remind yourself: “This is one question, not the whole exam.” Then move forward.
Divide the test into three mental zones (first 50, middle 50, last 50). Expect fatigue to peak in the middle—plan to stretch briefly, close your eyes, or breathe deeply after question 75.
Trust your preparation. Avoid second-guessing unless you clearly misread a key phrase. Doubt is often a sign of fatigue, not lack of knowledge.
These tactics condition you to stay focused, conserve energy, and recover faster from mental dips. They help ensure you don’t just start strong—but finish with clarity and control.
Get Certified Through CCRPS’s CRC Program
Most CRC exam guides focus on preparation strategies—but overlook the single most important factor influencing your success: the training program itself. If your certification provider doesn’t simulate real-world clinical research demands, or if it delivers outdated theory without context, no amount of effort will make up for it.
That’s why the CCRPS Clinical Research Coordinator Certification stands apart. It’s engineered not just to help you pass the exam, but to prepare you for real sponsor expectations, regulatory audits, and on-site decision-making—the actual role of a high-performing CRC. From GCP mastery to audit simulation, CCRPS’s program delivers global job readiness, not just test prep.
Below, we break down the full advantage of choosing CCRPS and how to enroll.
Benefits of CCRPS Certification
The CCRPS CRC program is trusted by research sites, CROs, and hospitals across 80+ countries for one reason: it focuses on competency-based learning. Every module trains you to act—not just memorize.
Here’s what makes CCRPS’s program different:
542+ Lessons Across 8 Core Domains
The course doesn’t skip over real-world pain points. You’ll dive into protocol deviations, AE/SAE documentation, site monitoring readiness, and protocol amendments with actual CRO templates and redacted sponsor cases.Global GCP + FDA + EMA Integration
While many programs focus only on local standards, CCRPS teaches you how to operate under international regulatory systems—crucial if you’re aiming for roles at global sites or multinational CROs.Simulated Sponsor-CRC Interaction Scenarios
You’ll complete simulations that place you in the role of the CRC facing protocol dilemmas, subject compliance challenges, or audit prep decisions. Each includes coaching notes and debriefs.Self-Paced, Exam-Aligned Structure
With flexible access and lifetime updates, you can complete the program on your own schedule—while receiving real-time alignment with the current CRC exam blueprint.CPD and ICH-GCP Accredited
The course is GCP accredited and globally recognized. Completing the CCRPS program adds weight to your resume and improves your competitiveness for high-paying roles.Built-In Practice Exams and Flashcard Banks
Instead of searching for separate prep tools, CCRPS includes scenario-based quizzes, domain-specific flashcards, and full-length timed mock exams within the program.
Graduates report up to a 92% first-time pass rate, thanks to this all-in-one, industry-vetted structure.
CCRPS Enrollment Details
Enrolling in the CCRPS Clinical Research Coordinator Certification is straightforward and fast. The process is designed to be fully online, allowing candidates from any region or time zone to start immediately.
Here’s what you can expect:
Visit the official course page: https://ccrps.org/clinical-research-coordinator-certification
You’ll find a detailed curriculum breakdown, accreditation proof, student reviews, and module previews.Choose your plan:
There are flexible payment options available, including installment plans and institutional reimbursement pathways for sponsored employees.Instant Access Upon Enrollment:
Once enrolled, you receive immediate access to the entire 500+ lesson structure, quizzes, and exam prep tools. No delays. No prerequisites.1-on-1 Instructor Mentoring Available:
You can opt-in for private mentoring, where senior CRAs walk you through scenarios, protocol samples, and compliance corrections.Exam Voucher Included:
Unlike many programs that charge separately, CCRPS includes the exam fee in the certification bundle—so there are no hidden costs.
With a single enrollment, you gain not only exam preparation but career-proof CRC skills that translate directly into your next role—be it at a sponsor site, SMO, hospital, or CRO.
Frequently Asked Questions
-
The most effective way to study for the CRC exam is to follow a structured, blueprint-based approach. Focus on high-yield topics such as informed consent, SAE reporting, site file maintenance, and GCP compliance. Use active recall with scenario-based flashcards, and test your reasoning through full-length mock exams that mirror the real format. Avoid passive reading and instead focus on doing—such as annotating protocols, filling mock CRFs, and answering regulatory case studies. Weekly assessments should track performance by domain. Pair this with spaced repetition to reinforce memory over time. The CCRPS Clinical Research Coordinator Certification program includes a ready-made system that aligns your prep with the actual exam blueprint—making the process more efficient and exam-specific.
-
Preparation time varies by experience, but most candidates need between 6 to 8 weeks of consistent study. For those new to clinical research, 10–12 weeks may be necessary. The key is not duration, but depth and structure. Use the exam blueprint to allocate time proportionally—more hours for regulatory guidelines and monitoring prep, less for general clinical operations. A strong prep plan includes daily review blocks (60–90 minutes), weekly simulations, and GCP scenario practice. Break topics into cycles to reinforce retention. The CCRPS program is self-paced, so you can adjust based on personal speed without falling behind. Whether you're working full-time or transitioning into research, aim for at least 100–120 focused study hours to confidently pass on your first attempt.
-
The CRC certification exam consists of 130–150 multiple-choice questions, delivered in a timed format of approximately 3 hours. Questions are heavily scenario-based, not just factual or definitional. You’ll be asked to apply GCP, FDA/EMA compliance, and sponsor-site procedures to real-life situations—such as dealing with protocol deviations, adverse events, and audit readiness. Only one answer is correct per question, and each domain (e.g., informed consent, monitoring prep, regulatory documentation) carries a weighted value. The exam may be proctored online or administered at an authorized testing center. The CCRPS CRC exam is available 100% online with flexible scheduling. Expect to navigate clinical vignettes, patient safety dilemmas, and documentation workflows—so theoretical understanding won’t be enough. Realistic practice exams are essential to succeed.
-
The CCRPS Clinical Research Coordinator Certification is designed by senior CRAs, site managers, and regulatory experts to meet global clinical trial expectations. Unlike other programs that rely on outdated textbooks or static PDFs, CCRPS offers 542+ lessons, real-world CRO templates, and simulated site tasks. Every module is aligned with ICH-GCP, FDA, EMA, and sponsor-level protocols, helping you master more than just the test. The program includes adaptive quizzes, scenario-driven flashcards, mock exams, and even 1-on-1 mentoring. Students get lifetime access with quarterly curriculum updates—something most competitors do not provide. With an industry-leading 92% first-time pass rate and CPD accreditation, CCRPS doesn’t just train you to pass the exam—it trains you to operate effectively as a CRC on day one.
-
Most certification providers allow a retake window, often within 30 to 90 days. However, retaking without understanding why you failed often leads to repeat attempts. First, review your mock exams—were you underprepared in certain domains (e.g., protocol deviation handling)? Did time pressure affect your judgment? The CCRPS platform includes analytics that tracks your performance by domain, helping you pinpoint gaps. If you're a CCRPS student, you’ll also receive continued access to quizzes, flashcards, and updated mock tests after your first attempt. In most cases, one structured revision cycle—targeting weak areas and simulating under timed conditions—will be enough to succeed. However, your focus must shift from memorization to logic-driven decision-making. That’s what the exam is truly testing.
-
Yes, the CCRPS Clinical Research Coordinator Certification is internationally recognized and used by students in 80+ countries. Its curriculum is aligned with ICH-GCP, FDA, EMA, and global regulatory standards, which means it’s relevant for research professionals working in sponsor sites, CROs, hospitals, and academic centers worldwide. While some countries may have local licensure or credentialing systems, the core principles of Good Clinical Practice and clinical trial coordination remain universal. CCRPS's CPD accreditation and ICH-GCP alignment ensure its acceptability in clinical research networks globally. If you’re planning to work outside your current country, this certification gives you cross-border credibility in multinational research settings—especially in high-compliance regions like the U.S., EU, Canada, UAE, India, and Australia.
-
Salaries for certified CRCs vary by region, experience, and employer type—but the presence of certification boosts entry-level and mid-career earnings significantly. In the U.S., CRCs with certification earn an average of $62,000 to $85,000 annually, with senior coordinators surpassing $95,000. In regions like the UAE, UK, or Singapore, salaries range from $40,000 to $70,000 USD equivalent, often with additional bonuses. Certification from a globally recognized provider like CCRPS signals that you're ready for sponsor/CRO-level expectations, increasing your value in the job market. Many employers now list certification as preferred or required, and some offer direct reimbursement for CCRPS enrollment. With demand growing for research staff worldwide, certified CRCs are positioned for fast career acceleration, remote opportunities, and site leadership roles.
-
Yes, the CRC certification exam through CCRPS is fully online and remotely proctored. You can schedule it at your convenience without needing to travel to a test center. Once enrolled, you’ll get immediate access to the platform’s exam simulator, mock tests, and prep resources. The online exam mimics the real-world testing environment: timed scenarios, multiple-choice logic questions, and randomized question order. A stable internet connection, webcam, and quiet testing area are all you need to take the exam from home or work. This makes it especially accessible to professionals in remote regions or those balancing full-time jobs. With built-in proctoring and result validation, your certification retains full credibility while offering maximum flexibility in scheduling and delivery.
Final Thoughts
Passing the CRC exam on your first attempt isn’t about luck—it’s about preparation that mirrors the real-world demands of clinical research. By understanding the exam structure, using evidence-backed study methods, avoiding common pitfalls, and practicing under simulated conditions, you put yourself in the strongest possible position for success. But the most strategic decision you can make is choosing a certification program that aligns with your goals and the industry's expectations.
The CCRPS Clinical Research Coordinator Certification gives you more than a credential—it gives you the skills, mindset, and confidence to thrive as a global clinical research professional. Whether you’re aiming for your first CRC role or leveling up within a CRO, this certification ensures you're not just ready to pass—you’re ready to lead.