Interactive Directory of Remote Patient Monitoring Tools for Clinical Trials
Remote patient monitoring (RPM) is no longer a future-facing concept—it’s a clinical trial essential. As decentralized and hybrid trials become standard, RPM tools like wearables, biosensors, and mobile apps now power real-time data collection across sites and continents. The old model of site-only visits can’t keep up with modern regulatory expectations or patient demand for flexibility.
This article is your strategic guide to RPM tools in clinical trials. You’ll discover what RPM includes, how it’s recognized by regulators like the FDA and EMA, and why it's mission-critical in today's decentralized trial designs. From digital biomarkers in wearables to AI-driven remote monitoring platforms, we break down what works, what integrates with your tech stack, and what to avoid. You’ll also learn how clinical research certifications now embed RPM as a core training area. Let’s get into the tools driving the next generation of trials.
What Is Remote Patient Monitoring in Clinical Trials?
Defining RPM and Its Clinical Trial Use Cases
Remote patient monitoring (RPM) in clinical trials refers to the use of digital tools like wearables, sensors, and mobile apps to collect patient data outside of traditional clinical settings. RPM systems enable continuous or scheduled monitoring of vital signs, physical activity, medication adherence, and symptom reporting—all in real time.
Unlike traditional site-based monitoring, which relies on periodic in-person visits, RPM captures a fuller and more granular picture of patient outcomes, including off-site safety signals and adherence behavior. This is especially critical in oncology, cardiovascular, and rare disease trials, where missing a single dose or adverse event can compromise protocol integrity.
RPM tools also reduce reliance on patient recall, replacing subjective self-reporting with objective biometric data. By integrating with electronic clinical outcome assessments (eCOAs) and electronic data capture (EDC) systems, RPM helps unify trial workflows while decentralizing execution.
Regulatory and Industry Recognition
Regulators globally now recognize RPM as a validated method of data capture in decentralized clinical trials (DCTs). The FDA’s Digital Health Policy outlines expectations for devices used in remote settings, and its 2023 draft guidance on DCTs encourages sponsors to use RPM to reduce site visits and improve trial accessibility.
The European Medicines Agency (EMA) also endorses the use of real-world data from RPM tools, particularly in post-market studies and long-term follow-up. Meanwhile, ICH E6(R3) modernizes trial oversight expectations, explicitly supporting risk-based monitoring and digital endpoints.
With decentralized trials expanding across therapeutic areas, RPM tools have moved from optional to mission-critical. Sponsors that ignore RPM risk slower enrollment, lower retention, and increased regulatory burden as data expectations evolve.
| Aspect | Details |
|---|---|
| Definition | Use of digital tools (wearables, sensors, apps) to collect patient data remotely during clinical trials. |
| Key Functions | Monitor vitals, activity, medication adherence, and symptoms in real time—outside of clinic visits. |
| Clinical Advantage | Provides granular, objective data; reduces patient recall bias and improves safety tracking. |
| System Integration | Connects with eCOAs and EDCs to streamline data flow and support decentralized workflows. |
| Regulatory Support | FDA, EMA, and ICH E6(R3) endorse RPM for decentralized clinical trials and digital endpoints. |
| Adoption Risk | Not adopting RPM may lead to slower enrollment, lower retention, and regulatory pushback. |
Key Categories of Remote Monitoring Tools
Wearable Devices
Wearables are the most recognized form of RPM. Devices like Apple Watch, Fitbit, and Garmin track physical activity, heart rate, and sleep cycles, while biosensors such as BioIntelliSense and VitalConnect capture continuous clinical-grade data. These tools are especially powerful in cardiovascular, neurodegenerative, and oncology trials, where long-term monitoring of vitals or movement is critical for endpoint analysis.
Advanced wearables support passive data collection, reducing patient burden and enhancing compliance. Some are FDA-cleared for clinical use, and most now integrate directly into ePRO and EDC platforms, streamlining real-time data transfer. Sponsors are increasingly using wearable-derived digital biomarkers to detect early signs of disease progression or adverse reactions long before traditional methods would reveal them.
Mobile Health (mHealth) Apps
mHealth apps serve as patient interfaces for ePRO reporting, symptom tracking, and compliance management. Platforms like Medable and uMotif offer guided workflows, automated reminders, and real-time clinician alerts, ensuring patients complete assessments on time while flagging any deviations from expected patterns.
They also play a key role in managing remote consent, scheduling, and bidirectional communication between patient and site, essential for maintaining engagement in long-duration studies.
Implantables and Ingestibles
Implantables and ingestibles represent the frontier of RPM. Smart pills like Proteus and implantable sensors enable the passive capture of physiological data and precise tracking of medication adherence. These tools are often used in studies requiring tight dosing control, such as transplant immunosuppressants or chemotherapy.
While regulatory hurdles are higher for these devices, their ability to capture unobtrusive, high-integrity data makes them increasingly attractive for complex trial designs.
Top Remote Patient Monitoring Platforms Used in Trials
Medidata Sensor Cloud
Medidata Sensor Cloud is one of the most robust and FDA-aligned RPM solutions in use today. Developed by Medidata (a Dassault Systèmes company), it supports integration with a wide range of sensor brands and is optimized for clinical-grade data ingestion. It connects directly with Medidata’s eCOA, EDC, and Rave Clinical Cloud, allowing real-time synchronization of patient data across platforms.
Its open framework enables sponsors to incorporate biosensor, wearable, and app data from vendors like BioIntelliSense and Garmin, maintaining fidelity and regulatory compliance. Medidata’s work with the FDA on standardizing digital endpoints gives it a unique edge in trials requiring validated remote monitoring workflows.
Science 37 RPM Suite
Science 37's RPM suite is built into its full-service decentralized trial platform, delivering end-to-end virtual trial capabilities. Its RPM tools allow real-time vitals monitoring, patient check-ins, and adverse event flagging via mobile and wearable integrations.
What sets Science 37 apart is its AI-powered alert system. It continuously analyzes patient data streams to identify early risk signals, improving safety monitoring without requiring site visits. The platform also enables rapid enrollment and better geographic diversity, making it ideal for global Phase II–IV trials.
Koneksa Health
Koneksa focuses on digital biomarker development, turning raw wearable data into FDA-acceptable endpoints. It specializes in respiratory, oncology, and neurodegenerative trials, using passive monitoring tools to create consistent, repeatable measures of disease progression.
Its differentiator lies in its scientific validation pipeline, offering a clear bridge between exploratory data and regulatory-grade submission material.
ActiGraph and VivoSense
ActiGraph and VivoSense are commonly used in academic and post-market studies. ActiGraph devices focus on sleep, activity, and movement patterns, while VivoSense offers advanced sensor analytics, including cognitive assessments and respiratory signals.
They’re valued for their granular timestamping and algorithmic flexibility, often chosen for studies where specific signal processing is critical to the protocol.
📊 Poll: Which RPM Platform Do You Trust Most in Clinical Trials?
Results will be updated weekly based on reader submissions.
Benefits of RPM for Sponsors, Sites, and Patients
Real-Time Data & Fewer Site Visits
RPM eliminates the latency of traditional data capture. Instead of waiting weeks for site visits, sponsors now receive continuous, real-time streams of patient data, enabling interim analysis, adaptive trial design, and faster decision-making. This level of responsiveness is especially critical in high-risk studies where dose adjustments hinge on moment-to-moment changes.
Patients benefit as well. With fewer on-site visits required, travel burdens are reduced—particularly important for elderly participants, rare disease populations, or those in rural locations. The convenience translates to higher retention, better protocol adherence, and more diverse participant recruitment.
Sites also see operational gains. Remote monitoring reduces the overhead of manual data entry and source verification. Research coordinators can focus on value-adding clinical tasks instead of redundant paperwork.
Better Safety Monitoring and Protocol Compliance
Continuous monitoring offers far more than convenience—it enhances safety oversight and protocol compliance. RPM tools can immediately flag vital sign changes, medication lapses, or adverse symptoms, triggering alerts to both site staff and central monitors.
This enables earlier detection of serious adverse events, allowing proactive intervention. The data also ensures compliance with procedural timelines, such as dosing windows and biometric assessments, and creates a digital audit trail aligned with regulatory expectations.
With clear timestamping and automated logging, RPM reduces the chance of missed entries, falsification, or ambiguity, strengthening the reliability of your clinical trial’s safety data.
For Sponsors
Real-time data flow enables interim analyses, faster decisions, and adaptive designs. RPM allows sponsors to act on clinical trends within hours—not weeks—enhancing agility in trial management.
For Sites
Fewer manual processes means CRCs spend more time on high-value clinical tasks. Remote verification automates audit trails and reduces paperwork overhead.
For Patients
Fewer site visits reduces travel burden and improves retention, especially for rural, elderly, and rare disease participants. RPM personalizes convenience without compromising data quality.
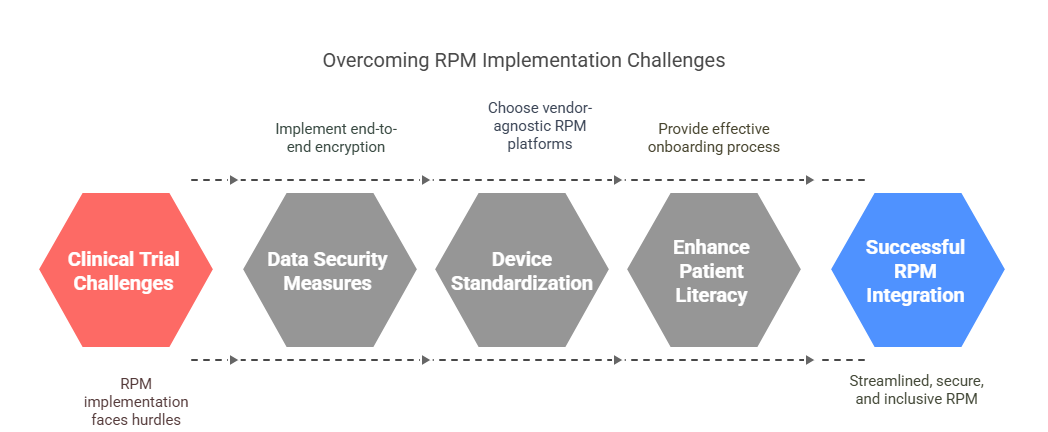
Challenges in Implementing RPM in Clinical Trials
Data Security and Privacy Compliance
RPM tools must comply with a complex patchwork of global privacy laws, including HIPAA, GDPR, and 21 CFR Part 11. Unlike traditional data capture methods, RPM involves constant transmission of sensitive health data over wireless networks, cloud platforms, and third-party APIs.
To mitigate risks, vendors must implement end-to-end encryption, biometric access controls, and multi-factor authentication. Identity verification protocols are essential to ensure data integrity and subject traceability. Audits should confirm that backup and breach response plans are in place, especially for cross-border trials involving data residency rules.
Failure to meet these standards can result in protocol rejection, data exclusion, or even legal penalties—making privacy-by-design a non-negotiable feature in RPM system selection.
Device Standardization and Integration Issues
RPM success hinges on device interoperability. Trials often require wearable, ingestible, or app-based tools to interface seamlessly with EDC, ePRO, CTMS, and eConsent systems. Yet many devices operate in siloed ecosystems, making integration a persistent challenge.
Standardizing data formats (e.g., HL7 FHIR, CDISC ODM) and choosing vendor-agnostic RPM platforms can alleviate compatibility issues. However, real-world deployment often reveals mismatches in sampling rates, data timestamping, or syncing errors that lead to protocol deviations.
This technical fragmentation forces sponsors to either limit their toolset or invest in costly middleware, increasing trial setup timelines and complexity.
Patient Digital Literacy and Adoption Barriers
Not all trial participants are comfortable using connected health devices. Age, education level, and geographic access to high-speed internet heavily influence RPM usability and adoption. For global trials, these disparities can introduce data inconsistency or attrition risks.
Effective onboarding, user-friendly interfaces, and real-time support are vital to bridge this digital divide. Sponsors that ignore these barriers risk noncompliance, dropouts, or biased datasets that compromise trial validity.
Remote Patient Monitoring Tool Selection Criteria
Key Questions for Trial Sponsors to Ask
Before committing to any RPM solution, sponsors must ask a set of non-negotiable strategic questions. The first: Is the platform compliant with regulatory standards? Ensure the tool supports HIPAA, GDPR, 21 CFR Part 11, and has documented audit trails and encryption protocols. If it doesn’t, the tool may not survive protocol review or site inspections.
Second: Does the RPM system align with your trial endpoints? A wearable that tracks movement may be useless in a cardiac trial requiring continuous ECG signals. Confirm that the platform is validated for your intended biomarkers, frequencies, and output formats.
Third: Is the tool ecosystem-agnostic? Many RPM tools are locked into proprietary apps or hardware. Vendor-agnostic platforms, on the other hand, allow you to switch sensors or add modules without overhauling your protocol. For multi-phase studies or adaptive trials, this future-proofing is critical.
Sponsors must also evaluate patient support infrastructure, scalability across sites, and multilingual interface availability. A powerful RPM platform that fails to scale globally or lacks 24/7 support will collapse under pressure.
Integration With Trial Tech Stack
RPM tools are not standalone products—they must seamlessly integrate with the broader trial tech stack. At minimum, the system must be compatible with electronic data capture (EDC), clinical trial management systems (CTMS), eConsent, and ePRO platforms. Preferably, this integration should be real-time and API-based, not dependent on manual file uploads or CSV transfers.
Sponsors should verify that the RPM tool handles protocol versioning, country-specific privacy rules, and allows for modular customization based on site capabilities. The ideal platform supports drag-and-drop configuration, enabling rapid deployment across heterogeneous research environments.
Integration also determines data latency, reconciliation effort, and endpoint reliability. Poorly integrated tools slow down the trial, burden sites, and risk noncompliance. Prioritize platforms built for the full clinical ecosystem—not just patient engagement.
Remote Patient Monitoring Tool Selection Criteria
Confirm support for HIPAA, GDPR, and 21 CFR Part 11. Look for secure audit trails, encryption, and clear documentation.
Ensure the RPM tool can collect validated biomarkers relevant to your trial’s outcomes and data frequency needs.
Choose platforms that support multiple device brands and allow module swapping without major protocol changes.
Look for multilingual interfaces, international support teams, and infrastructure that scales across trial regions.
The system should integrate with EDC, CTMS, eConsent, and ePRO platforms using modern APIs—avoiding manual data entry.
Select tools that offer rapid customization and deployment across heterogeneous site capabilities and evolving protocols.
Explore RPM Inside Our Clinical Research Certification Program
Remote patient monitoring (RPM) is no longer optional—it’s a core competency in modern clinical research. As trials decentralize and regulatory scrutiny intensifies, sponsors now expect clinical research professionals to understand how to select, deploy, and interpret data from RPM tools. That’s exactly what our certification delivers.
Through the CCRPS Advanced Clinical Research Associate (CRA) Certification, students master the full spectrum of RPM integration—from evaluating wearable devices and biosensors to analyzing real-time safety alerts and adverse event trends. The program includes hands-on training with leading RPM platforms, compliance modules covering HIPAA and GDPR, and practical case studies involving decentralized oncology and cardiology trials.
We go beyond theory. Our learners work with real-world trial simulations that explore how RPM is used to reduce patient burden, enhance data integrity, and improve recruitment in underserved regions. You'll also learn how to interface with data teams, manage protocol adaptations, and support remote site monitoring with high compliance visibility.
Whether you’re advancing your CRA career or expanding into global trial oversight, this certification gives you the RPM knowledge clinical sponsors now demand.
Final Thoughts
Remote patient monitoring tools have rapidly evolved from experimental add-ons to mission-critical infrastructure in clinical trials. As decentralized and hybrid models become the new norm, RPM is no longer a competitive advantage—it’s a regulatory and operational necessity. Sponsors, CROs, and sites that embrace these tools benefit from faster data cycles, improved safety oversight, and significantly better patient retention.
For clinical research professionals, staying ahead means mastering the technology stack driving today’s trials. Whether it’s evaluating device interoperability, understanding privacy-by-design frameworks, or interpreting real-time vitals, RPM fluency is now a required skillset. Those who lead in this space will shape the future of trial innovation, execution, and regulatory trust.
Frequently Asked Questions
-
Remote patient monitoring (RPM) tools in clinical trials include any digital device or software that collects patient data outside of the traditional clinical setting. This spans wearables like smartwatches, biosensors for continuous vitals, mobile health apps for symptom tracking, implantables, and even ingestibles that report dosing adherence. The key criterion is that the data must be medically relevant, securely transmitted, and ideally integrated with the trial’s data ecosystem—such as EDC or ePRO platforms. Tools must also comply with regulatory standards like HIPAA and 21 CFR Part 11 to qualify for use in FDA-regulated or EMA-reviewed studies.
-
Sponsors start by aligning platform capabilities with trial endpoints and regulatory requirements. The tool must support the collection frequency, data type, and validation level required by the protocol. Next, they assess whether the RPM system is vendor-agnostic, globally scalable, and integrable with the existing trial tech stack (EDC, CTMS, eConsent). Security is also non-negotiable—platforms must offer encryption, audit logs, and role-based access controls. Finally, ease of patient use is a decisive factor, as user-friendly tools yield better compliance, higher retention, and cleaner data.
-
Yes—both the FDA and EMA recognize RPM as valid data sources, especially for decentralized and hybrid trial models. The FDA’s Digital Health Policy and 2023 draft DCT guidance explicitly encourage RPM use for reducing site visits and increasing data granularity. Similarly, the EMA accepts real-world data from RPM tools, particularly when it supports post-authorization safety and effectiveness studies. Both agencies require data traceability, security, and validation of the devices, and increasingly look for evidence of patient-centric design and regulatory-grade audit readiness in RPM systems.
-
RPM can collect a wide range of objective and subjective health metrics, including heart rate, blood pressure, respiratory rate, oxygen saturation, physical activity, sleep quality, and glucose levels. Tools like mHealth apps can also capture patient-reported outcomes (ePROs), medication adherence logs, and symptom diaries. Implantables may gather internal biomarkers or pharmacokinetic signals. Some RPM tools are designed for continuous data streams, while others capture data at specific intervals based on protocol needs. The diversity of data supports adaptive trial designs, risk-based monitoring, and more nuanced safety analysis.
-
Key barriers include data privacy compliance, device interoperability issues, and patient digital literacy. Many tools fail to integrate with existing clinical trial systems, creating fragmented workflows and delays. Sponsors also struggle with selecting globally scalable platforms that meet varying privacy laws like GDPR and HIPAA. On the patient side, older adults or those in low-bandwidth regions may face access issues. Without proper onboarding, real-time support, and intuitive UX design, RPM can lead to dropouts or inconsistent data capture, undermining trial integrity.