Clinical Research Assistant: A Complete Guide to Becoming A CTA with No Experience on Resume
How To Become A Clinical Research Assistant
The Ultimate 2025 Guide To Becoming A Clinical Trial Assistant With No Experience On Resume
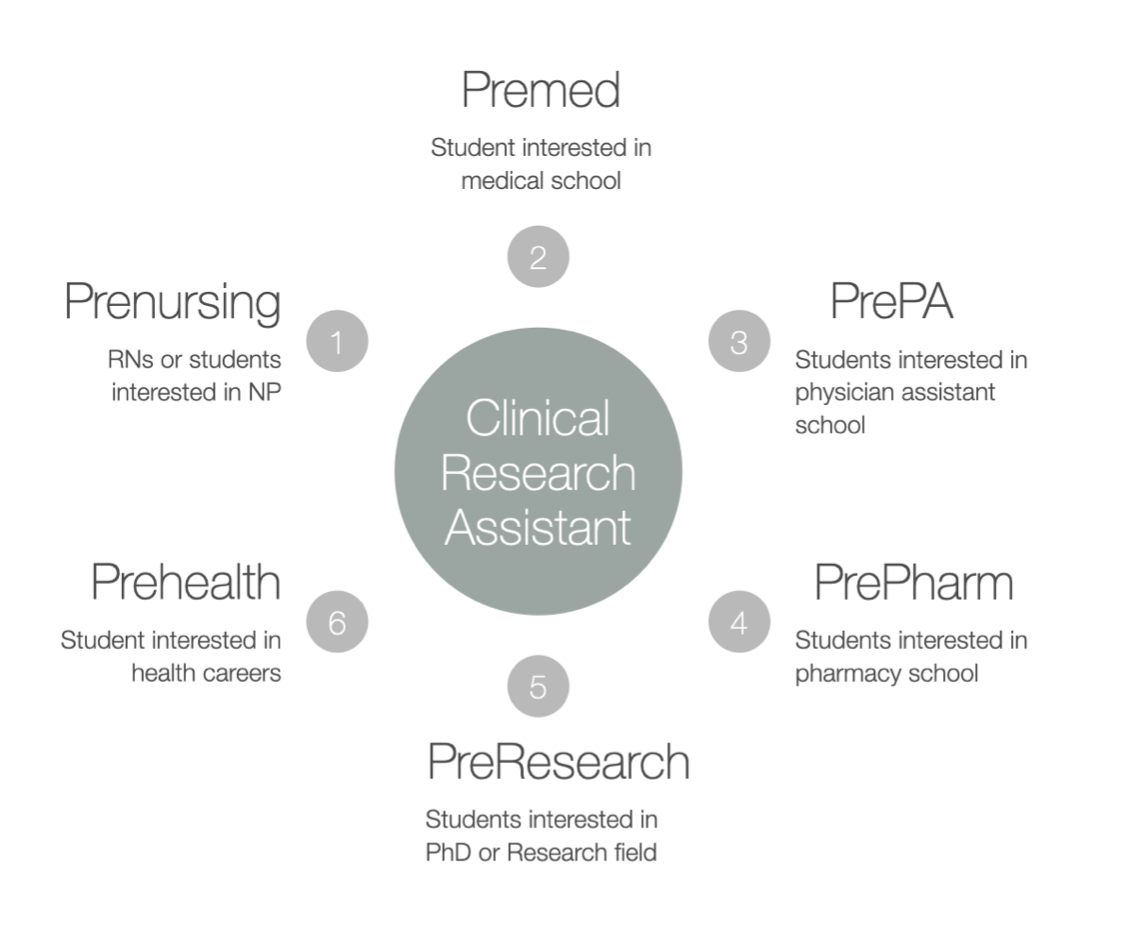
See the pathways to clinical research assistants
Clinical Research Assistant
Is a Career as a Clinical Research Assistant Right for You?
The role of a Clinical Research Assistant (CTA) in clinical research is both crucial and rewarding. CTAs are integral to the success of clinical trials, ensuring they run smoothly, ethically, and effectively. If you're considering a career in this dynamic field, ask yourself:
Are you ready to take on greater workplace responsibilities?
Can you safeguard the safety and well-being of trial participants?
Do you thrive in organized environments where attention to detail is key?
If you find yourself answering "Yes!" to these questions, you may be perfectly suited for this exciting and impactful career.
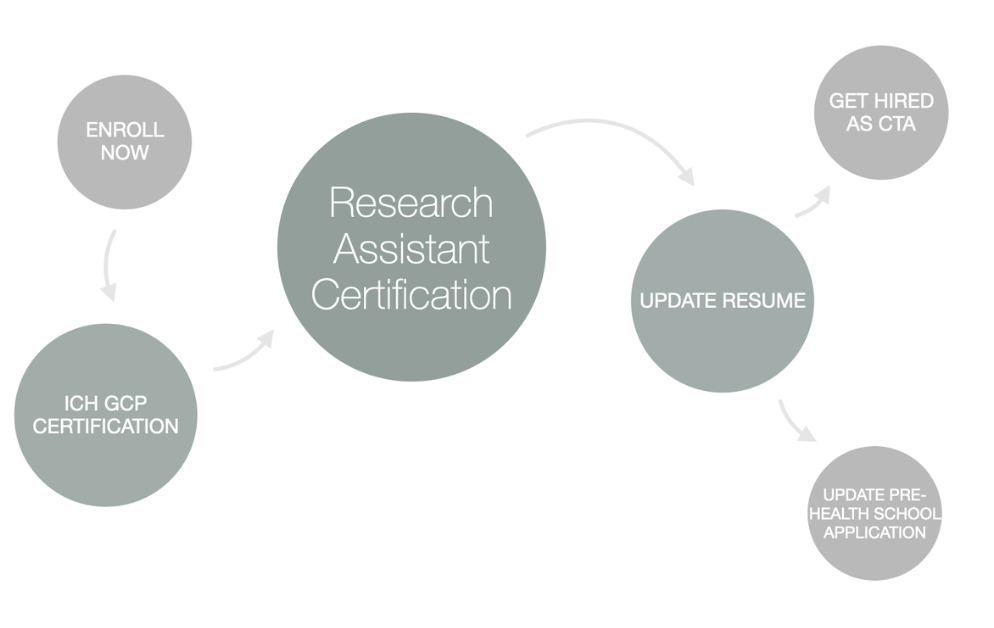
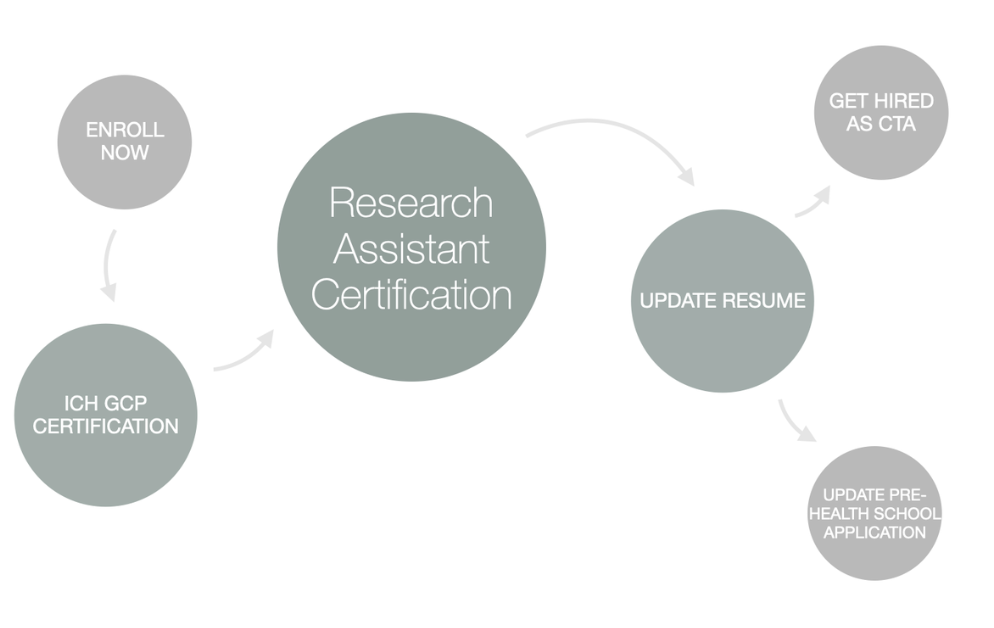
To improve your chances of success and accelerate your career, CCRPS offers the only accredited, on-demand Advanced Clinical Research Assistant Certification (ACTAC). This flexible, 3-week program equips you with the skills and knowledge to stand out when applying for jobs and thrive once you're hired.
Why Choose a Career as a Clinical Research Assistant?
Being a Clinical Research Assistant goes beyond just having a job—it’s about driving innovation in healthcare. From monitoring trial participants to ensuring compliance with ethical standards, CTAs play a pivotal role in research that improves lives. This career offers both personal fulfillment and the opportunity for professional growth.
Whether you're just starting or looking to enhance your current role, CCRPS's Advanced Clinical Research Assistant Certification is designed for success. This program helps you:
Build in-depth knowledge of clinical research procedures.
Improve your employability by earning an industry-recognized certification.
Develop confidence in managing the diverse responsibilities of a CTA.
What Are the Responsibilities of a Clinical Research Assistant (CTA)?
A Clinical Research Assistant (also called a Clinical Trial Assistant or CTA) is an essential part of clinical research teams. Their work ensures clinical trials are conducted efficiently, ethically, and in compliance with regulations. If you're considering this career, understanding the day-to-day responsibilities is key to preparing for the role.
Here’s a clearer look at what CTAs do and why their role is so important:
Core Responsibilities of a Clinical Research Assistant:
Maintain Standard Operating Procedures (SOPs): SOPs act as the rulebook for clinical trials. CTAs are responsible for ensuring these procedures are followed accurately to maintain compliance and consistency throughout the trial.
Provide Regular Reports: CTAs keep everyone informed by providing detailed reports on the progress of studies. These updates help the research team make informed decisions, track results, and stay on schedule.
Conduct Pre-Study Evaluations: Before trials begin, CTAs help assess potential study sites. They review these locations to ensure they meet all requirements for conducting safe and effective clinical trials.
Evaluate Site Feasibility: CTAs determine whether a site has the proper capabilities to conduct the planned study. This might involve assessing available resources, staffing, and patient populations.
Monitor Study Adherence: Perhaps one of the most critical tasks, CTAs are responsible for making sure every aspect of the trial adheres to regulatory standards and clinical protocols. This safeguards participant safety and ensures the trial meets outlined objectives.
Clinical Research Assistant Job Description
When applying for a Clinical Research Assistant role, it’s helpful to understand what prospective employers expect. Below is a snapshot of a typical job description for this position:
Trial Design and Oversight: Assist in the planning, coordination, and administration of clinical trials. This includes managing specific trial tasks under the guidance of supervisors while ensuring compliance with goals and timelines.
Analyze Clinical Data: CTAs review data collected during trials to ensure it aligns with the trial’s objectives. This requires attention to detail and knowledge of research guidelines.
Ensure Regulatory Compliance: Every trial must meet requirements set by regulatory bodies like the FDA. CTAs help ensure data collection and trial procedures align with these standards.
Collaborate with Team Members: CTAs work closely with Clinical Research Coordinators (CRCs), Principal Investigators (PIs), and site staff to complete trials successfully.
Required Skills and Qualifications
Clinical research is a specialized field, and CTAs must meet certain qualifications to succeed. Most employers look for candidates with the following:
A BS, RN, or BSN degree (or equivalent), though some positions accept high school diplomas with relevant experience.
0–3 years of experience in clinical research or a related area.
A clear understanding of FDA regulatory requirements and clinical research protocols.
Familiarity with commonly used concepts, practices, and procedures in the field.
The ability to follow detailed instructions and predefined guidelines.
Career Growth and Impact
Being a Clinical Research Assistant isn’t just a job—it’s a role that makes a tangible difference. Every responsibility you take on contributes to bringing new treatments to patients safely and efficiently. Plus, the experience and skills you develop as a CTA can open doors to advanced positions, like Clinical Research Coordinator (CRC) or Clinical Research Associate (CRA).
Minimum Education Requirements for a Clinical Research Assistant
For most entry-level Clinical Research Assistant roles, employers look for candidates who meet the following educational criteria:
Minimum Requirement: High school diploma or equivalent.
Preferred: An associate degree or bachelor's degree (B.Sc.) in a health-related field such as nursing, life sciences, medical science, or biotechnology.
If you don't have a degree specific to health sciences, coursework or experience in related areas, like biology or chemistry, can make a strong impression. If applicable, make sure to highlight these qualifications when applying.
An alternative pathway to becoming a Clinical Research Assistant is through certification. Certification programs, like the Advanced Clinical Research Assistant Certification (ACTAC) by CCRPS, can help you develop the specialized knowledge and administrative skills needed to excel in this field. They are particularly beneficial for those without formal education in health sciences.
Essential Skills for a Strong Clinical Research Assistant Resume
Your skills are just as important as your education when applying for a Clinical Research Assistant role. Here are the key abilities that employers value most in this position:
Knowledge of database management challenges: Understand how to implement, maintain, and troubleshoot research databases.
Comprehensive understanding of clinical protocols: Be familiar with the ethical and legal requirements for human trials and data handling.
Clinical development planning: Demonstrate the ability to design effective and practical clinical development strategies.
Attention to detail in data accuracy: Ensure trial data is precise, reliable, and adheres to participant privacy laws.
Organizational and time-management skills: Coordinate trial-related tasks while managing multiple priorities.
Mastering these skills not only makes your job as a CTA easier but also ensures that you’re performing your role efficiently and ethically.
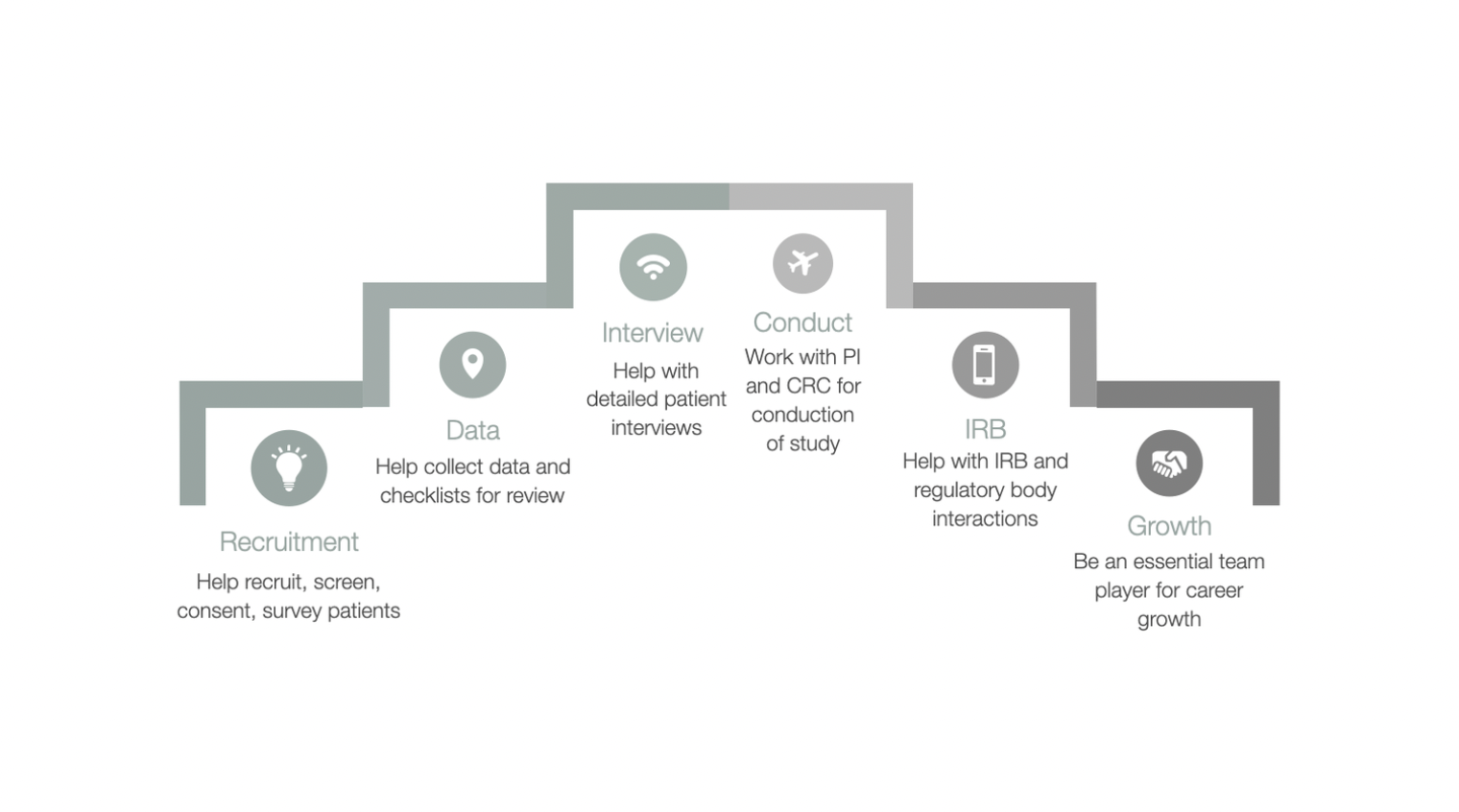
Core Responsibilities of a Clinical Research Assistant
A Clinical Research Assistant wears many hats and plays an integral role in ensuring clinical trials are conducted smoothly and effectively. Below are the core responsibilities you can expect in this role:
Conduct literature reviews to provide context for research projects.
Collect, analyze, and ensure the accuracy of trial data.
Prepare materials for grant applications and submissions.
Design interview questions, recruit participants, and summarize interviews while maintaining confidentiality.
Monitor study sites to ensure compliance with study protocols and ethical guidelines.
Maintain records, request supplies, and supervise junior team members when necessary.
Prepare project reports, presentations, and other materials for senior staff.
Travel to sites to collect data and provide technical support where required.
Assist with quality control, track project progress, and contribute to the development of study protocols.
Resume and Cover Letter Tips
When applying for a Clinical Research Assistant role, showcasing your qualifications, skills, and experiences effectively in your application materials is vital. Here are some tips to help you stand out.
Resume Examples and Tips
Your resume should highlight your education and relevant work or volunteer experience in a clear and concise format. Focus on incorporating the following sections:
Contact Information: Full name, email address, phone number, and LinkedIn profile link (if applicable).
Professional Summary: A two-to-three-sentence overview of your experience and skills as they relate to clinical research. For example, “Detail-oriented Clinical Research Assistant with a strong background in data analysis, protocol management, and participant recruitment.”
Skills: Include essential skills such as compliance monitoring, data management, attention to detail, and knowledge of FDA regulations.
Education: List your highest degree first, including certifications such as the ACTAC certification from CCRPS, if applicable.
Experience: Highlight your relevant roles, describing duties with action verbs such as “coordinated,” “monitored,” “prepared,” and “analyzed.” Use bullet points to make this section easy to scan. Be sure to quantify your achievements, such as by noting the number of trials you supported or the percentage of accurate data you contributed.
Cover Letter Tips
A tailored cover letter allows you to expand on your resume and demonstrate your enthusiasm for the role. Follow this structure:
Introduction: Briefly share why you’re excited about the role and the organization. For example, “With a passion for advancing healthcare innovation, I am thrilled to apply for the Clinical Research Assistant role at [Organization Name].”
Skills and Experiences: Provide specific examples of how your background aligns with the job’s requirements. Mention relevant coursework, certifications, or experiences.
Key Achievements: Highlight accomplishments that underscore your ability to contribute immediately to the research team.
Closing Statement: End with a confident call to action, such as, “I would welcome the opportunity to discuss how my skills and experience align with your team’s goals. Please find my resume attached for review.”
If you're aiming to impress, using action-packed examples and showcasing results can help your application resonate with hiring managers.
Your Path to Success as a Clinical Research Assistant
Obtaining the right education, honing essential skills, and presenting a polished and professional resume and cover letter are your first steps toward building a successful career as a Clinical Research Assistant. Remember, certifications like the ACTAC by CCRPS can help you stand out in this competitive field.
A Step-by-Step Guide to Finding Trial Assistant Experiences and Internships
Breaking into clinical research can feel challenging, but the right guidance and proactive steps can significantly ease the process. Clinical Trial Assistant internships and experiences are stepping stones to a rewarding career, where you'll contribute to advancing healthcare. This guide will walk you through practical steps, resources, and strategies to help you land your first opportunity.
Get ready to take action, boost your confidence, and chart a path toward an exciting future in clinical research!
Step 1: Build a Strong Foundation with Education and Certification
While a degree in life sciences, nursing, or health sciences is an asset, it’s not the only route into clinical research. Certification programs can often be the game-changer, particularly if you’re transitioning into this field from another profession.
Here’s why certification matters:
It demonstrates your commitment to pursuing a career in clinical research.
It equips you with the fundamental knowledge and skills employers value.
It increases your chances of standing out in a competitive job market.
Programs like the CCRPS Clinical Trials Assistant Training prepare you with practical skills in patient safety, informed consent, trial management, and ethical compliance. Certification gives you a head start by making your resume shine and showing employers you’re ready for real-world responsibilities.
Already certified? Even better! Use that experience to show how you're ready for hands-on opportunities.
Step 2: Find Trial Assistant Positions Where It Matters
Your next step is to figure out where employers in clinical research are looking for candidates like you. Here are the top resources to get you started:
1. Specialized Job Boards
Focus on platforms dedicated to clinical research professionals for high-quality and targeted opportunities.
SOCRA Job Board and ACRP Job Board: Regularly post internships and entry-level roles for CTAs.
Clinical organization websites often have specific “Careers” sections—check them out directly!
2. General Job Boards
Popular sites like LinkedIn and Indeed are full of research assistant internships. Here’s how to make them work for you:
Use keywords like “Clinical Trial Assistant Internship,” “Research Assistant,” and “Clinical Research Coordinator” to filter your search.
Set email alerts to be the first to know about openings.
Pro Tip: Don’t skim over temporary or project-based positions—they’re fantastic for gaining experience and building connections.
Step 3: Leverage University Resources (Even if You’re No Longer a Student)
If you're currently studying or recently graduated, universities can be goldmines for opportunities.
1. Career Services Departments
These teams specialize in matching students with internships. Schedule an appointment to discuss your career goals, and they’ll help you identify potential opportunities.
2. Research Departments
Many universities conduct clinical trials, often led by professors or Ph.D. candidates, who need assistance. Don’t hesitate to email professors or program coordinators to ask about any open positions—they may not be publicly advertised!
Example email:
“Dear [Professor’s Name],
I’m [Your Name], a [student/recent graduate] interested in gaining hands-on experience in clinical research. I noticed your department is involved in [specific research/project], and I’d love to contribute as an assistant or intern. Please let me know if there are any opportunities to support your team.”
Step 4: Tap into Government Resources
Regulatory Agencies:
Organizations like the FDA and NIH sometimes offer formal student programs or volunteer roles in clinical research. Check out their websites, as they often list ongoing programs ideal for gaining real-world exposure.
You can also explore nationwide clinical trial directories like ClinicalTrials.gov, which lists trials by type, location, and coordinating organizations. Follow up with those conducting trials in your city to see if they need assistance.
Step 5: Build & Use a Professional Network
Networking isn’t just about finding opportunities; it’s also about learning more about the field and connecting with mentors.
1. Join Professional Associations
Memberships with groups like SOCRA or ACRP give you access to networking events and job postings. They often host online webinars or local meetups, where professionals offer advice and mentorship.
2. Optimize LinkedIn Connections
Don’t stop at creating a profile—use LinkedIn actively!
Join clinical research groups and participate in discussions.
Send connection requests to active professionals or recruiters, along with tailored messages explaining your goals and interest in the industry.
Need a script? Here’s one to start with:
“Hi [Name],
I’m actively pursuing opportunities as a Clinical Trial Assistant and came across your inspiring profile. I’d love to learn about your career path and any advice you have for someone new to this field.”
Step 6: Volunteer for Experience
Volunteering isn’t only a way to give back—it’s a great way to gain firsthand insight into clinical trials.
Hospitals and Research Institutions: Offer your assistance to teams conducting trials. Sometimes, all it takes is expressing interest and a willingness to learn.
Patient Advocacy Groups: These organizations often interact with clinical research groups and may know of openings, as advocacy is closely tied to trials.
Step 7: Tailor Your Application Materials
To stand out, don’t use a generic resume and cover letter. Instead:
Match keywords to the job description. This ensures your application passes any initial automated screenings.
Show passion. Hiring managers appreciate applicants who are genuinely excited about the role. Include specific career goals that relate to the organization.
Highlight relevant skills. Even if they’re not from clinical research (think multitasking, handling data, or strong written communication).
Pro Tip: Include your CCRPS certification prominently on your resume to signal your expertise and boost your chances of snagging an interview.
Step 8: Be Proactive and Persistent
Internships and entry-level roles aren’t always advertised. Don’t wait—reach out directly to companies conducting trials. Here’s how to approach them confidently:
Research local CROs, biotech firms, or pharmaceutical companies using directories like BioPharmCatalyst or CRO Directory.
Send targeted emails outlining your interest and the value you bring.
Certification Is Your Secret Weapon
Earning certification as a Clinical Trial Assistant through programs like CCRPS sets you apart from the pack. Here’s why students love this program:
Practical Knowledge: From trial management to patient engagement, you’ll master job-ready skills.
Career Boost: Past students frequently credit certification for landing their first dream roles.
Flexible & Affordable: CCRPS offers partial scholarships, flexible payment plans, and 24/7 advisor support to make advancement accessible for anyone motivated to succeed.
Don’t miss out on this opportunity to strengthen your qualifications. Learn more about the CCRPS Clinical Trials Assistant Training today!
Your Next Step Starts Here
Putting yourself out there can feel intimidating, but every message sent, application submitted, or certification earned brings you one step closer to a fulfilling career in clinical research.
Follow these actionable steps to build your resume, gain real-world experience, and set yourself apart. By prioritizing networking, leveraging resources, and investing in certification, you’ll be ready to take on this exciting field with confidence.
Remember, your dream role is waiting—you just have to take the first step. The future of clinical research starts with you.
Why wait? Enroll in the CCRPS Clinical Trials Assistant Training Course today and put yourself on the fast track to success!
Advanced Clinical Trial Assistant Training Syllabus
Welcome to the Advanced Clinical Trial Assistant Training Program!
Are you ready to take your clinical research career to the next level? This comprehensive, hands-on course is designed to prepare you to excel as a Clinical Trial Assistant (CTA), equipping you with the skills, knowledge, and confidence to make a real impact in the world of clinical research. Whether you're new to the field or aiming to sharpen your expertise, this syllabus will give you a sneak peek into the exciting topics you'll explore in this training program.
Start Here
Introduction
Kick things off with an overview of the course and the professional standards upheld by the Accreditation Council for Clinical Research & Education for CCRPS. You'll learn how this certification sets you apart in the clinical research industry.
Master the Fundamentals of Clinical Research
1. An Introduction to Clinical Research
Discover what makes clinical research the backbone of modern medicine. This section introduces you to the principles and processes that drive innovative treatments.
2. Understanding ICH GCP Guidelines
Unpack the international gold standard for clinical research—and learn why these guidelines are vital for ensuring ethics, quality, and compliance in trials.
3. Code of Federal Regulations (CFR)
Take a detailed look at CFR 21 Part 11, which governs the use of electronic systems in clinical research. Gain insights into navigating regulatory frameworks with ease.
Clarify Clinical Trial Roles and Responsibilities
1. Sponsor and CRO Responsibilities
Step into the shoes of the key players in clinical trials! Learn what drives sponsors, CROs, and investigators while maintaining compliance with ethical standards.
2. The 13 Principles, IRB Oversight, and Investigator Roles
Explore the key roles and responsibilities behind clinical trials and see how the Institutional Review Board (IRB) ensures the safety and well-being of participants.
3. Informed Consent and Patient Safety
Master the critical process of informed consent to protect patient rights while ensuring clarity and compliance.
Champion Safety in Clinical Research
1. Protecting Human Subjects in Clinical Research
This module is all about keeping participants safe. Get a closer look at the ethical and procedural guidelines that form the backbone of every clinical trial.
2. Adverse Event Reporting
Take control of adverse event reporting by exploring the roles and responsibilities of investigators. Learn how to document and handle adverse events to uphold safety and compliance.
Tackle Ethics in Research
1. Researching Vulnerable Populations
Discover strategies to ethically and safely conduct clinical trials involving vulnerable groups, including children, pregnant women, mentally incapacitated individuals, and prisoners.
Level Up Your Trial Management and Data Handling
1. Keep Your Research Seamless
From trial management to record retention, learn how to organize, handle, and safeguard critical data—skills that will make you stand out in any research team.
Common terminology and abbreviations in clinical research
Data handling best practices
Dive Into Advanced Clinical Trials Knowledge
1. Deep-Dive into Clinical Trial Designs
Explore advanced trial designs and how they optimize drug development processes. This module lets you peek under the hood of what it takes to create innovative trials.
2. Phases of Clinical Trials – Preclinical to Phase 4
Go beyond the basics with an advanced breakdown of all clinical trial phases, with real-world insights into preclinical research and beyond.
➤ FREE PREVIEW: Get your first look at this exciting and comprehensive module!
Understand the Power of Patient Engagement
1. Recruitment, Retention, and Compliance in Clinical Trials
Patient-centric research is the future! Learn how to recruit, engage, and retain participants while ensuring they adhere to trial requirements. These strategies are critical for trial success in a competitive landscape.
Strengthen Your Integrity in Research
1. Preventing Misconduct and Fraud
Dive into the serious issue of scientific misconduct, learn to detect falsifications, and build your skills to ensure research integrity at every step of the way.
Certification Exam – Show What You’ve Got!
1. ICH GCP Clinical Trials Assistant Certification Exam
Test your newfound skills and knowledge with a 30-question exam designed to certify your expertise. Passing this exam proves you’ve mastered the art of managing clinical trials ethically and effectively!
What To Know For Clinical Trial Assistant Interview Questions
The work of a clinical research assistant is one of extreme importance to the clinical research institute, and employers will to testing to see if you understand what position entails.
Discover the Exciting World of Clinical Research – Why Becoming a Clinical Research Assistant is Your Next Move
Imagine a career where your work directly contributes to groundbreaking medical advancements—new treatments, life-saving medications, and cutting-edge medical devices. Sounds thrilling, doesn’t it? That’s exactly what it means to be a Clinical Research Assistant (CRA). If you’re someone who loves details, thrives in a fast-paced environment, and wants to make an impact on global healthcare, keep reading—you’re in for an exciting career path!
And here’s the best part: with the right training, you can step into this rewarding role quickly and confidently.
What’s a Clinical Research Assistant?
Think of CRAs as the superheroes behind medical research. They work behind the scenes to ensure clinical trials—used to test new drugs and medical devices—are safe, efficient, and compliant with regulations. Every data point, participant interaction, and test result goes through the critical hands of a CRA to guarantee accuracy, safety, and ethical compliance.
The work involves a mix of organization, science, and teamwork. You’ll do everything from finding participants for trials to analyzing data, ensuring studies are running smoothly, and keeping all the documentation in check. Without CRAs' dedication to the details, clinical trials simply wouldn’t happen.
And here’s the kicker—it’s a HIGH demand field with endless opportunities to grow.
A Day in the Life of a Clinical Research Assistant
Being a CRA is far from monotonous. Every day is different, which makes the job as dynamic as it is fulfilling. Here’s a snapshot of what your workday might look like:
Morning Safety Checks: You’ll kick off the day by checking essential equipment like freezers storing trial samples or medications. Why? Because proper conditions are critical to research validity, and even a tiny deviation can mess things up. By doing this, you’re not just following a routine—you’re ensuring years of hard work don’t go to waste!
Finding Trial Participants: Imagine recruiting volunteers who might one day benefit from treatments resulting from your work. You’ll screen participants, explain processes, and make sure they’re a perfect fit for the trial.
Data Collection and Review: If you’re someone who loves precision, you’ll thrive here. You’ll ensure the data is collected accurately, organized perfectly, and matches the strict regulations required for medical research.
Team Collaboration: Need a break from the details? You’ll work side-by-side with scientists, doctors, and sponsors, helping them understand the progress of the trials and addressing any concerns.
One thing’s for sure—you’ll never be bored. Plus, the impact of what you do is far-reaching and extraordinary.
Why This Career is in Demand
Clinical trials are essential for advancing medicine, and CRAs make those trials possible. Because of this, demand for skilled CRAs is skyrocketing, and so are the opportunities to work in top-tier organizations.
Pharmaceutical Companies are always on the lookout for CRAs to assist with life-saving drugs.
Biotech Companies need experts to help bring revolutionary treatments like gene therapy to life.
Hospitals and Academic Research Centers rely on CRAs to explore cutting-edge care methods.
Whether you want a role focusing on lab work, patient interaction, or project management, there’s a place just waiting for you as a CRA.
Oh, and did we mention that CRAs also enjoy great salary growth? Early in your career, you could make $40,000–$55,000 per year, with potential to reach $75,000+ as you gain experience.
What Does It Take to Be a Great CRA?
If you’re thinking, “Could I really do this?”, the answer is YES—if you bring the right skills and mindset. Here’s what makes a successful CRA:
Attention to Detail – Are you the kind of person who notices the small, important things others might overlook? Perfect!
Strong Communication Skills – You’ll collaborate with diverse teams, explain complex ideas, and share important study information.
Multitasking Mastery – From data entry to equipment checks, you’ve got to juggle a bit. Think of it as keeping all the puzzle pieces together while building something incredible.
Curiosity and a Desire to Learn – Science evolves rapidly, and staying ahead of the curve makes you stand out.
Good news? You can develop these skills further with the right training. That’s where programs like the CCRPS Clinical Trials Assistant Training course come in.
Why Training Sets You Apart
Here’s the secret to breaking into this high-demand field and thriving—the right training. Sure, you could try figuring it out on your own, but why not take the fast track with an expert-led program that gives you everything you need from day one?
The CCRPS Clinical Trials Assistant Training is tailored to help aspiring CRAs build the exact skills employers want. You’ll walk away with confidence, a respected certification, and the knowledge you need to stand out in a crowded job market.
What you’ll learn in the course:
Master the ICH GCP guidelines that govern global clinical trials.
Learn essential compliance, safety, and quality check protocols.
Build expertise in trial management tools used in real research facilities.
Gain practical knowledge to confidently manage participants, data, and compliance.
The best part? Employers take your certification seriously because it proves you’re ready to hit the ground running. With your credentials, you’ll save them weeks of training time—which means they’d rather hire YOU.
Step Into a Career That Saves Lives
If you’re ready to start a career that’s as meaningful as it is rewarding, the time to act is NOW. Every day, Clinical Research Assistants drive innovations that save lives and rewrite medical possibilities. And with demand growing, there’s no better time to step into this exciting field.
Equip yourself with the skills you need to get started and succeed. The CCRPS Clinical Trials Assistant Training course offers everything you need to enter confidently, elevate your career faster, and make an impact in healthcare that you’ll be proud of.
Don’t wait. Enroll today and take the first step toward becoming a leader in clinical research.
Clinical Research Assistant Salary
Per Payscale
Comprehensive Guide to Clinical Research Assistant (CRA) Salary
Clinical Research Assistant Salary — A Path to Growth
A career as a Clinical Research Assistant (CRA) is not only rewarding but also offers exciting growth opportunities, both professionally and financially. Understanding the salary dynamics in this field can help you plan your career and take actionable steps to maximize your earning potential. Whether you’re starting fresh or looking to advance, this guide provides everything you need to know about CRA salaries, influencing factors, and how to boost your career prospects.
Average CRA Salary Breakdown
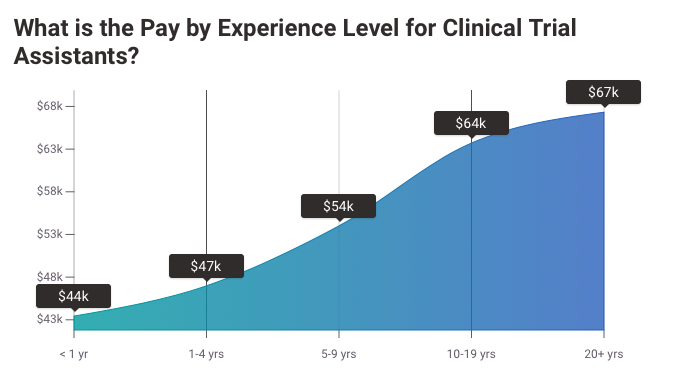
Clinical Research Assistants typically earn $40,000–$55,000 annually, with variation based on experience, education, certifications, and geographic location. Here's a snapshot of salaries across different experience levels:
Entry-Level (0–2 years): $35,000–$45,000 annually.
Mid-Level (3–5 years): $45,000–$60,000 annually.
Senior-Level (5+ years): $60,000–$75,000+, especially if advancing to coordinator or associate roles.
For those working part-time or on contract, hourly rates range from $17 to $25/hour, often higher in cities with higher costs of living.
Key Factors That Influence CRA Salaries
1. Education and Certification
Your educational background plays a significant role in determining salary. Holding a bachelor’s degree in life sciences, healthcare, or related fields provides a competitive edge. But it’s certifications that turbocharge earning potential.
Certifications, such as those offered by CCRPS Clinical Trials Assistant Training, have been shown to increase starting salaries by as much as 20%. These programs go beyond traditional degrees to equip candidates with ICH GCP guidelines, ethical research protocols, data management, and trial compliance expertise.
Investing in a certification not only validates your skills but can also position you as a standout candidate for higher-paying positions.
2. Experience Levels
Experience holds the key to unlocking higher salaries. Entry-level CRAs gain essential exposure to trial support roles, such as data collection and patient monitoring, which builds the foundation for growth. Senior professionals with expertise in managing complex trials or overseeing regulatory compliance earn premium compensation.
3. Location and Demand
Where you work matters. Geographic location influences salaries due to demand, cost of living, and proximity to research hubs.
High-paying states in the U.S.:
California ($55,000–$75,000 annually)
Massachusetts ($50,000–$70,000 annually)
North Carolina ($48,000–$65,000 annually, especially near major CROs like IQVIA).
If you’re willing to relocate, consider metropolitan cities such as Boston, San Francisco, or Raleigh-Durham, as they often have the highest demand for CRAs.
4. Employer Type
CRAs employed by pharmaceutical companies or Contract Research Organizations (CROs) tend to earn more compared to roles in academia or non-profits. Here’s what to expect based on employers:
Pharmaceutical/Biotech Companies: $60,000–$75,000 annually.
Contract Research Organizations (CROs): $50,000–$65,000 annually.
Hospitals and Universities: $40,000–$55,000 annually.
5. Specialized Skills
Having niche skills can make all the difference. High-demand proficiencies such as statistical programming (Python, SAS), experience with trial management tools like REDCap, and expertise in oncology trials or precision medicine often come with better salaries.
Fun Fact: CRAs with proficiency in patient recruitment and adverse event reporting frequently earn top-level pay because of the critical role these skills play in trial success.
Strategies to Maximize Your Earnings
1. Get Certified
Obtaining certification is one of the fastest ways to strengthen your resume and negotiate higher salaries. The CCRPS Clinical Trials Assistant Training program is specifically designed to prepare you for lucrative career opportunities with specialized training in clinical trials management and compliance.
2. Gain Real-World Experience
Internships, volunteering at trial sites, or part-time roles offer invaluable experience. Start small, focus on building your skillset, and use this to leverage better positions as you grow.
3. Build a Network in Major Research Hubs
Networking within research centers, CROs, or pharmaceutical giants in cities like Boston or San Diego can fast-track your growth. Many positions come through referrals, so building relationships in the industry is critical.
4. Transition to Advanced Roles
Set your sights on transitioning to roles like Clinical Research Coordinator (CRC) or Clinical Research Associate (CRA). These positions often pay $75,000–$100,000 annually and involve more leadership responsibilities.
Pro Tip:
Highlighting success in managing trials, ensuring regulatory compliance, or improving patient retention metrics can increase your value within any organization.
Global Salary Perspectives
If you’re considering opportunities outside the U.S., here’s an overview of CRA salaries globally:
United Kingdom: £25,000–£40,000 annually, with senior roles hitting £60,000.
Canada: CAD 47,000–CAD 80,000 annually.
Australia: AUD 60,000–AUD 85,000 annually.
India: Entry-level CRAs earn ₹3,00,000 to ₹5,00,000 per year.
While salaries differ globally, ICH GCP certification ensures you stay competitive regardless of the market.
Final Thoughts
Clinical Research Assistant salaries offer promising growth if you focus on building relevant qualifications, experience, and skills. Pave the way for your advancement by investing in specialized training like the CCRPS Clinical Trials Assistant Training.
CTA Salary Prediction Provided by Payscale
CCRPS offers the only clinical research assistant certification (ACRAC) course available
Step Into the Exciting World of Clinical Research
Thinking about a career that’s meaningful, rewarding, and full of opportunities? Becoming a Clinical Research Assistant (CRA) might be the perfect fit for you. Not only can it boost your earning potential, but it also gives you the knowledge and confidence to thrive in clinical research—an essential field in healthcare.
Start Your Journey
If you’ve got a bachelor’s degree in life sciences or social sciences, you’re already on your way! But don’t worry if you don’t. Enrolling in a relevant bachelor’s program and volunteering for clinical trials can help you gain valuable experience and open doors to this exciting career.
What Is Clinical Research?
Clinical research is all about testing new drugs, therapies, and medical devices to ensure they’re safe and effective. These trials are tightly regulated and monitored to make sure everything is done ethically and safely. But all this work creates a mountain of data and compliance requirements—and that’s where Clinical Research Assistants step in.
What Do Clinical Research Assistants Do?
Think of Clinical Research Assistants as the backbone of clinical trials. They manage things like:
Finding and working with trial participants.
Collecting and analyzing data.
Keeping detailed records to meet compliance standards.
They’re the ones who ensure the entire trial runs smoothly and stays on track with legal and ethical requirements. Because of their key role, these professionals are in high demand!
Endless Opportunities
One of the coolest things about being a Clinical Research Assistant is the variety of places you can work. You could find yourself working in:
Research institutes
Hospitals or medical centers
Pharmaceutical companies
Biotech firms
Wherever you land, you’ll play a part in groundbreaking medical advancements.
What Qualifications Do You Need?
For this career, most employers look for a bachelor’s, master’s, or doctorate degree in life sciences, medical sciences, or a related field. But beyond your degree, hands-on knowledge and experience are what really make you stand out.
This is why certifications, like the Advanced Clinical Research Assistant Certification (ACTAC) by CCRPS, are so important. These programs teach you the ins and outs of clinical research, giving you an edge when applying for jobs.
Why Should You Get Certified?
Even if you have a degree, a targeted certification like ACTAC can help you stand out in a competitive field. With this certification, you can:
Build core skills in clinical research.
Boost your chances of getting hired.
Be more effective and successful in your role.
And the best part? The ACTAC course is on-demand, so you can complete it in just three weeks at your own pace.
Take Action Today
If a career in clinical research excites you, don’t wait to get started. Invest in your education, learn from experts, and gain real-world experience. CCRPS offers everything you need to succeed, from cutting-edge courses to expert mentorship.
Your efforts today could lead to tomorrow’s medical breakthroughs. Take the first step towards a fulfilling career as a Clinical Research Assistant by enrolling in CCRPS’s ACTAC certification course.
This is your moment—start your clinical research career today!
Triage Definition: The Ultimate Guide to Understanding The Meaning of Triage (Definition & Practices) - 2025 Edition
Triage is a term that resonates across multiple disciplines, from healthcare and emergency management to business strategy and IT systems. But what exactly does it mean, and why is it so important in critical decision-making? This guide unpacks the definition of triage, highlights its applications, and explores its relevance through engaging examples and actionable insights.
This structured and engaging guide includes 3 resourceful tables and practical strategies to help you master the concept of triage—whether you're a healthcare professional, project manager, or business leader.
What Is Triage?
At its core, triage is the process of prioritizing tasks, resources, or individuals based on the urgency and severity of need. Derived from the French word trier, meaning "to sort," triage is fundamentally a system for effective decision-making during resource scarcity or high-stakes scenarios.
The Three Pillars of Triage
Prioritization: Deciding which cases or tasks are the most urgent.
Resource Allocation: Determining how to efficiently use limited tools, personnel, or time.
Continuous Evaluation: Reassessing priorities as conditions evolve.
Triage ensures that the most pressing needs are addressed first, ultimately limiting risk and achieving optimal outcomes.
Triage in Various Fields
Triage is a universal concept, extending beyond healthcare to encompass fields like emergency management, IT troubleshooting, and business. Here’s how triage works in different sectors:
How Triage Works in Healthcare
The Three-Category Triage System
Healthcare professionals use triage primarily to prioritize patients in emergency care or disaster settings. Here is a breakdown of the commonly employed three-category triage system:
Immediate (Red): Critical cases requiring life-saving interventions (e.g., cardiac arrest).
Delayed (Yellow): Serious but not life-threatening; treatment can be postponed slightly (e.g., fractures).
Minor (Green): Non-urgent cases requiring basic treatment or observation (e.g., minor cuts).
Table 2. Healthcare Triage Categories
This structured categorization allows medics to focus care where it is most needed.
Actionable Tips for Triage Application
The effectiveness of any triage system depends on striking the right balance between urgency, resource capability, and outcomes. Follow these actionable steps to optimize the triage process.
Steps for Effective Triage
Establish Clear Criteria: Define what qualifies as high, moderate, and low priority. Use objective measures to prevent bias.
Integrate Technology: Tools like resource management software or automated alert systems improve efficiency and trackability.
Train Your Team: Conduct simulations to prepare staff for high-pressure decision-making scenarios.
Transitioning Your Skills to Work in Clinical Research
If you’re working in a field that relies on triage—such as healthcare, project management, or IT—you already possess valuable skills for transitioning into clinical research. Here’s how to leverage your existing expertise to build a career in this growing industry.
Why Clinical Research?
Clinical research plays a vital role in advancing medicine, and it requires professionals with exceptional organizational, analytical, and problem-solving skills. Transitioning into clinical research offers the chance to blend competitive salaries with meaningful contributions to healthcare innovation.
Key Transferable Skills
You might not realize it, but common triage-related skills are highly valuable for clinical research roles like Clinical Research Associates (CRA), Data Managers, or Regulatory Specialists.
Actionable Steps to Transition
Gain Certification: Get certified through programs like CCRPS to gain credibility and foundational knowledge. Certification not only provides the necessary competencies but also ensures regulatory readiness for clinical trials.
Highlight Your Experience: On your resume, emphasize how skills like prioritization and resource allocation from your current role are directly applicable to trial monitoring and patient safety.
Network Effectively: Join clinical research communities or forums to connect with professionals, access opportunities, and learn the industry's nuances.
Assess Your Readiness: Take the CCRPS Certification Quiz to determine how ready you are to step into clinical research and identify areas of improvement.
Take Free CCRPS Career Quiz Today
The Meaning of Triage: Triage in Pharmacovigilance and Clinical Research
Triage in Clinical Research
Clinical research is a dynamic field where new findings shape future healthcare innovations. While “triage” might recall an emergency room setting, it also plays a critical role in research management. Triage in clinical research is essential for prioritizing the enrollment of participants, optimizing resources, and driving impactful results. This guide unpacks the meaning of triage, its applications in clinical trials, and actionable strategies to ensure impactful collaboration and outcomes.
For professionals looking to expand their clinical research expertise, this guide also provides direct links to certifications and training programs to propel your career forward on platforms like app.ccrps.org.
What Is Triage in Clinical Research?
Modern clinical research adopts triage as a systematic sorting process, where potential participants or trial aspects are assessed and prioritized based on urgency, eligibility, and trial objectives. This mirrors medical triage’s goal of addressing critical needs first but adapts it to focus more on enrollment and decision-making efficiency within the research ecosystem.
Key Goals in Clinical Research Triage
Efficient Participant Selection: Identifying individuals who meet specific eligibility criteria ensures resources aren't wasted, and trials proceed smoothly. To improve your expertise in this area, check out the Clinical Research Coordinator Course.
Maximizing Trial Success: Prioritizing optimal matches between participants and trial objectives improves meaningful outcomes. Learn more about best practices for maintaining trial safety in the Pharmacovigilance Certification.
Triage Steps in Clinical Trials
Step 1. Determine Eligibility
Eligibility serves as the foundation of clinical research triage. Factors such as diagnosis, age, medical history, and treatment status are scrutinized to align participant profiles with trial goals.
Screening Tools: Digital systems like EHRs (Electronic Health Records) streamline identifying eligible participants.
Certification Opportunity: Discover how Clinical Trials Assistant professionals handle eligibility processes through specialized training.
Step 2. Patient Randomization
Eligible participants are divided into treatment and control groups. This step ensures impartiality and strengthens the trial's scientific validity.
Tip: Deepen your understanding of controlled environments in trials by earning the ICH-GCP Certification.
Step 3. Safety and Monitoring
Participants undergo regular monitoring for adverse events (AEs) while researchers evaluate the safety profile of investigational drugs or devices.
Learn More: Improve skills in monitoring trial safety by exploring the Medical Monitor Certification.
Step 4. Final Evaluation
The trial’s success depends on assessing whether the treatment significantly improved outcomes compared to the control.
Benefits of Triage for Researchers and Patients
Triage provides multifaceted advantages that improve process efficiency while delivering positive patient outcomes.
For Researchers
Streamlined Resource Use: Triage ensures researchers allocate time, patient slots, and funding to maximize trial impact.
Improved Collaboration: An organized triage process fosters teamwork among coordinators, regulatory officers, and medical monitors. Interested in facilitating better collaboration? Check out the Advanced Research Project Manager Certification.
For Patients
Priority Access: Patients most in need of treatment are enrolled first, ensuring they benefit from cutting-edge therapies.
Customized Care: Through rigorous selection and follow-ups, patients receive care personalized to their conditions.
Tips for Effective Triage in Pharmacovigilance
Triage also plays a critical role in post-market surveillance and pharmacovigilance. Here are actionable tips for clinical research professionals responsible for monitoring drug safety.
Categorize Potential Risks: Use a risk matrix to prioritize adverse drug events (ADEs) by severity, likelihood, and population impact.
Utilize Alert Systems: Automate and centralize reporting systems for ADEs to identify safety trends faster. Access tools with the Pharmacovigilance Certification.
Continual Training: Equip all team members with up-to-date training on drug safety monitoring. Explore options through ICH-GCP Certification.
Beyond Proficiency—The Importance of Collaboration
Knowing the nuances of triage is only half the equation. To truly excel, professionals must master collaboration across clinical teams. From physicians and nurses to data managers, ensuring consistent communication and shared understanding is critical for seamless trial execution.
Recommended Certification
Enhance collaboration skills by exploring the Clinical Research Associate (CRA) Course.
Empower Your Clinical Research Journey
Triage stands as the backbone of clinical trials, providing structure to participant selection, resource allocation, and adverse event management. For ambitious professionals aiming for leadership roles or specialized skills, certifications on app.ccrps.org offer the perfect stepping stone into advanced career opportunities.
Key Certifications to Accelerate Your Growth:
Clinical Trials Assistant Training: Ideal for support roles in trial execution.
ICH-GCP Certification: Gain comprehensive knowledge of international clinical trial guidelines.
Advanced Principal Investigator Physician Certification: Tailored for medical professionals aspiring to lead trials.
By mastering the art of clinical triage, you'll help shape the future of medicine—one trial at a time. Begin your transformational career today by exploring all the available programs and quizzes at app.ccrps.org.
The Steps of Triage in Clinical Trials
Triage in clinical trials is a structured process that ensures the efficient use of resources, while keeping patient safety and trial success at the forefront. Here is a step-by-step look at how triage functions in this context:
1. Determine Eligibility
The first and most critical step in clinical trial triage is establishing eligibility. Researchers evaluate participants based on specific criteria such as age, medical history, condition severity, and treatment status. These factors ensure that only individuals who align with the trial’s objectives are enrolled.
Why It Matters: Screening participants saves time and prevents resources from being spent on ineligible cases. It helps trials stay focused and efficient.
Tools & Training: Many trials now incorporate Electronic Health Records (EHRs) to verify eligibility. Explore more about trial coordination with the Clinical Research Coordinator Course.
2. Safety Assessment
The next step involves continuous monitoring of trial participants to safeguard their health. Researchers look for potential adverse events (AEs) or any sign of harm stemming from the investigational drug or treatment.
Action Plan:
Serious adverse events result in immediate suspension of treatment for the affected participant.
Medical care is provided promptly to address any observed risks.
Reliable safety assessment not only protects participants but also prevents regulatory issues that could halt the trial. Build expertise in safety monitoring through the Medical Monitor Certification.
3. Evaluate Efficacy
Finally, researchers assess how well the drug or treatment worked compared to placebo or standard care. This step determines whether the treatment has achieved its intended goals with minimal side effects.
Outcome: If the data points to safety and success, the treatment may move one step closer to regulatory approval.
Building Efficiency with Triage
Triage in clinical trials ensures that each phase—eligibility, safety, and efficacy—is properly executed. For those aspiring to play a pivotal role in carrying out these steps, the ICH-GCP Certification provides valuable insights into global clinical trial standards.
How Triage Prioritizes Patients in Clinical Research
Prioritizing patients in clinical research is both an art and a science. By sorting participants based on their level of need and suitability for the study, triage allows researchers to focus on the individuals most likely to benefit from treatment—and, in turn, contribute to meaningful outcomes. Here’s what that process looks like in detail:
Factors That Influence Triage Decisions
Severity of Illness: Patients with life-threatening or advanced conditions often get priority in trials designed for urgent interventions.
Resource Availability: Trials have limited resources, from study slots to medical staff, requiring careful prioritization to maximize efficiency.
Study Type: Some trials are intentionally designed to recruit only the most severe or uncommon cases.
Existing Patient Data: Patients with prior records of similar treatment can be excluded to avoid redundancy.
Key Insight: Those patients deemed high-risk or ineligible are excluded not to deny them care, but to ensure their safety, as some treatments could amplify risks.
Benefits of Triage in Clinical Trials
Triage brings substantial benefits for both researchers and patients, fostering an environment of streamlined operation and improved outcomes.
For Researchers
Efficient Resource Management: Triage ensures that time, funding, and personnel are used where they will make the greatest impact.
Faster Data Collection: By enrolling the most eligible patients, researchers gather higher quality data in a shorter time span.
Enhanced Collaboration: Clinical staff, data scientists, and regulators work seamlessly when decision-making is guided by triage principles.
Learn More: Build your collaborative skills with the CRA Course.
For Patients
Priority Access to Treatment: Patients with critical needs gain timely inclusion in trials offering life-changing therapies.
Personalized Care: Researchers ensure each patient receives the care that aligns best with their condition.
Improved Health Outcomes: Triage helps patients participate in well-structured trials with strong safety protocols.
Tips for Effective Clinical Triage in Pharmacovigilance
Efficient triage in clinical trials doesn’t end at enrollment. It extends into pharmacovigilance—monitoring the safety profile of new and approved drugs. Here are some key strategies to optimize triage in this critical phase:
1. Rank Adverse Events by Severity
Categorize potential adverse events (ADEs) based on their seriousness, likelihood, and population impact. Focus on the most urgent cases first.
2. Automate Reporting Systems
Use centralized digital platforms to flag ADE trends. Early detection prevents risks from escalating.
3. Promote Continued Training
Equip every team member with up-to-date training in pharmacovigilance to maintain a seamless workflow. Check out the Pharmacovigilance Certification to enhance your readiness.
Why Triage Matters
Triage isn’t just a method—it’s a philosophy guiding better decision-making in clinical research. By optimizing participant selection, ensuring safety, and fostering collaboration, triage enhances not just the quality of trials but also the lives of those they touch.
Whether you’re looking to extend your clinical research skills or refine your expertise in pharmacovigilance, now is the time to act. Gain certifications that align with your career goals by visiting app.ccrps.org today
Clinical Trial Coordinator: Guide to the Roles and Duties of a Clinical Trial Coordinator
A Clinical Trial Coordinator (CRC) is essential to the success of any clinical trial. Tasked with managing research activities at clinical sites, CRCs ensure compliance with protocols, Good Clinical Practice (GCP) guidelines, and regulatory requirements. These professionals bridge the gap between key stakeholders, including sponsors, Ethics Committees, and investigators, while prioritizing the integrity of trial processes and the safety of participants.
The role is multifaceted—combining organization, technical know-how, ethical judgment, and a strong commitment to excellence. Given the criticality of their functions, comprehensive training, such as CRC certification, is indispensable for excelling in this field. Below, we offer a detailed exploration of a CRC’s roles, responsibilities, tools, and opportunities for growth.
CRC Responsibilities Across Trial Phases
The responsibilities of a CRC span distinct trial phases, each requiring unique skills and attention to detail. By breaking these down, we can illustrate the diverse nature of their work:
1. Pre-Trial Responsibilities
Before a clinical trial begins, the CRC plays a crucial role in ensuring the research site is ready to meet ethical, regulatory, and operational standards. This phase involves extensive documentation, liaising with key stakeholders, and managing logistics.
Key Tasks Include:
Study Feasibility Assessments:
Oversee completion of questionnaires submitted by sponsors or CROs to determine the site’s suitability.
Provide logistics details (e.g., investigator credentials, infrastructure capacity).
Assist in finalizing the site selection process through pre-study site visits.
Ethical and Regulatory Preparation:
Collate and submit documentation for Ethics Committee approval, which includes the investigator CVs, study protocols, insurance certifications, and patient diaries.
Schedule investigator meetings to align all parties and ensure the site is prepared for trial initiation.
Training and Readiness:
Conduct onboarding for site staff to ensure familiarity with trial requirements.
File documents such as signed protocols, blank CRFs, and clinical trial coordinator certifications.
Example Scenario:
Considering a study testing a novel cancer drug, a CRC may work alongside oncologists to ensure the site meets advanced treatment administration capabilities, procure legal documentation, and carry out pre-site inspection visits with CRO representatives.
2. Responsibilities During the Trial
Once the trial starts, the CRC moves into an operational phase. This involves managing participants, ensuring data accuracy, and maintaining compliance with the protocol.
Key Tasks Include:
Participant Recruitment and Management:
Screen potential participants against inclusion and exclusion criteria.
Obtain informed consent, ensuring participants fully understand the trial procedures and risks.
Schedule visits and coordinate participant timelines to align with protocol requirements.
Data Oversight:
Use tools like IVRS/IWRS to randomize participants and document visit information.
Maintain detailed case report forms (CRFs) and ensure prompt submission to sponsors or Data Monitoring Committees (DMC).
Monitoring Adverse Events:
Record adverse events (AEs) and serious adverse events (SAEs), carefully documenting details such as drug dosage, administration route, and reactions.
Communicate all safety concerns to the Principal Investigator (PI) and sponsor for review and action.
Managing Medications and Equipment:
Oversee the storage and management of investigational products (IPs), ensuring compliance with pre-defined temperature and safety protocols.
Collaborate with site pharmacists to maintain drug accountability logs.
Example Scenario:
For a clinical trial involving wearable health monitors, a CRC may train participants on proper use, troubleshoot device issues, and ensure collected data aligns with the study protocol.
3. Post-Trial Responsibilities
The CRC’s work doesn’t end when the last participant visit is completed. The close-out phase is equally vital to ensure the trial meets archival and auditing standards.
Key Tasks Include:
Trial Close-Out:
Assist sponsors and CRAs during site close-out visits, resolving any documentation discrepancies.
Review and organize trial data in preparation for audits.
Document Archival:
Prepare study documents for long-term storage, ensuring regulatory compliance for retention periods (often 15-20 years).
Maintain updated inventories of trial documentation to respond swiftly to inquiries during follow-ups.
Additional Areas of Impact
Beyond the standard phases, CRCs have roles that require understanding of communication, technology, and industry trends.
Communication Skills and Stakeholder Management
Effective communication is at the core of a CRC’s responsibilities. They serve as a bridge between investigators, site staff, sponsors, and Ethics Committees.
Internal Team Coordination: CRCs foster collaboration across the clinical site team to ensure smooth implementation of the trial protocol.
Sponsor Relationships: These professionals maintain regular reporting with sponsors or CROs, offering updates on site progress and addressing challenges.
Example:
If a trial suddenly requires re-negotiating the clinical research agreement, a CRC’s communication skills will support seamless adjustments without delaying the trial.
Technological Tools in Clinical Research
Technology is integral for efficient trial management. CRCs use various tools to monitor compliance, record data, and enhance trial operations.
Data Management Tools: Electronic Data Capture (EDC) systems like REDCap or Medidata streamline documentation and reporting.
Participant Support: Telehealth tools expand recruitment efforts and assist with remote monitoring in decentralized trials.
Ethics and Problem-Solving
CRCs regularly encounter situations requiring sound ethical judgment. Whether managing protocol deviations or ensuring participant well-being, their decision-making has a lasting impact on trial success.
Example:
If a rural participant faces transportation challenges, a CRC may work with sponsors to arrange alternative solutions, such as remote assessments.
Career Opportunities and Certification Advantage
The demand for Clinical Trial Coordinators is increasing alongside the global growth of clinical trials. Certification offers multiple benefits, including:
Enhanced Skill Validation: CRC certification demonstrates expertise in trial management.
Career Progression: Suitable roles include Clinical Research Associates (CRAs), Project Managers, or Regulatory Specialists.
Global Opportunities: Proficiency in GCP standards enables CRCs to work internationally, adapting to diverse regulatory environments.
Why Choose CCRPS for Certification?
At CCRPS, we offer tailored certification programs to prepare you for success in clinical research.
Comprehensive Training: From CRC certification to ICH GCP training, our courses focus on critical skills such as ethical considerations, protocol implementation, and regulatory compliance.
Flexibility and Accessibility: Self-paced modules allow you to balance training with ongoing work.
Advanced Expertise Options: Explore Advanced Clinical Project Manager Certification or Principal Investigator Certification for accelerated career growth.
Recommended Courses:
Clinical Research Coordinator Certification: Ideal for aspiring CRCs looking to master trial management.
Pharmacovigilance Training: Deepen your understanding of drug safety and adverse events monitoring.
CRA Training: Perfect for CRCs seeking a transition into a monitoring role.
Take Action Today
Empower your career with advanced training from CCRPS. Whether you're starting in clinical research or aiming for career advancement, our certifications provide the edge you need.
Start your CRC certification today!
Clinical vs Laboratory Research: A Comprehensive Comparative Analysis for 2025 – CCRPS
Clinical and laboratory research remain foundational in the evolution of modern medicine, each contributing uniquely to healthcare innovation. While clinical research focuses on human subject trials to evaluate the safety and efficacy of medical interventions, laboratory research emphasizes uncovering scientific principles and mechanisms through basic research. For 2025, both fields are expected to undergo significant advancements, driven by technology, regulatory shifts, and evolving healthcare priorities.
Want to Work In Clinical Research Instead? Take 3 Question Career Assessment I View Certifications
The Evolution of Clinical and Laboratory Research
Clinical Trials and Research Advancements
Clinical research has progressed by becoming increasingly patient-centric, incorporating state-of-the-art technologies. The transition from traditional in-person trials to decentralized clinical trials (DCTs) has become a hallmark of modern research. By including wearable devices, real-world data collection, artificial intelligence (AI), and telemedicine tools, researchers now strive for greater inclusivity and efficiency in medical studies.
Laboratory Research Advancements
Laboratory research has evolved to offer unprecedented precision. Applied research takes findings from basic research and develops them into solutions that address specific market needs. Innovations include high-throughput screening, CRISPR gene editing, and advanced single-cell analysis. Cloud-based analytics and AI integration are driving faster discoveries, reducing the gap between hypothesis formation and medical application. Emerging fields such as synthetic biology and bioinformatics continue to push research boundaries, offering more targeted and scalable solutions.
Table 1: Key Differences Between Clinical and Laboratory Research in 2025
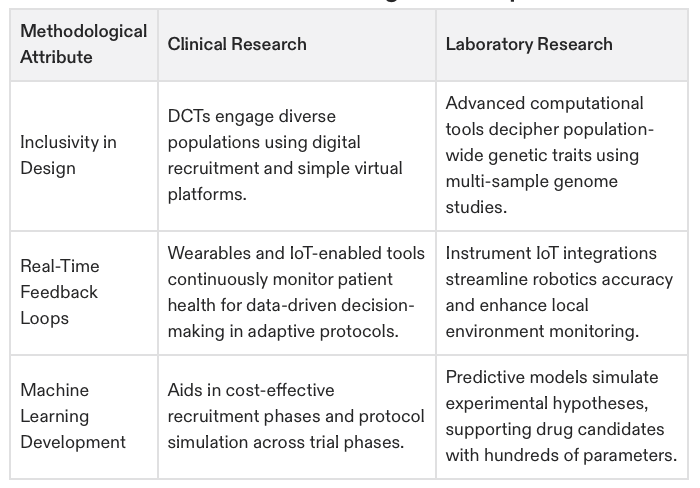
Emerging Methodologies
Clinical Research in 2025
Technological advancements are making trials more accessible, accurate, and efficient.
Patient Recruitment: AI algorithms are now identifying eligible patients across large datasets, drastically improving recruitment times.
Data Collection: Real-time monitoring through wearable devices provides continuous insights into participant health.
Trial Models:
Decentralized Trials enable remote participation, reducing geographical barriers.
Adaptive Trials allow protocol modifications based on interim results, increasing efficiency.
Laboratory Research in 2025
Laboratory research methodologies now balance advanced technology with methodological rigor.
Automation:
Robotic systems can simultaneously manage thousands of samples, enabling high-throughput workflows.
Advanced Modelling:
Computational tools powered by machine learning (ML) simulate outcomes with greater accuracy.
Multi-Omics Integration:
Combining genomics, proteomics, and metabolomics helps identify disease-specific biomarkers.
A structured approach to analyzing errors and conducting experiments can lead to improved methodologies that minimize errors and ensure systematic investigation in research processes.
Table 2: Methodological Comparison in 2025
Bridging the Gap through Collaboration
For 2025, increasing collaboration between clinical and laboratory researchers, where basic research is directly related to its applications in translational research, remains a primary force driving innovation. Enabling technologies such as cloud-based data systems, AI platforms, and cross-disciplinary training are breaking barriers between these two fields.
Key Collaboration Challenges
Data Integration:
Clinical data is often structured and dependent on human variability, while laboratory data is highly controlled. Bridging these requires advanced harmonization.
Communication Barriers:
Misaligned terminologies can hinder interdisciplinary teams from leveraging collaborative potential.
Solutions for Collaboration in 2025
Unified Data Platforms facilitate seamless data sharing between labs and clinical researchers, ensuring both teams work from compatible datasets.
AI-Driven Translational Pathways map molecular discoveries to real-world therapeutic applications.
Cross-Functional Training builds understanding between laboratory and clinical researchers, fostering effective teamwork.
Innovative solutions, such as digital workflows, can enhance the efficiency and continuity of clinical and laboratory research.
List 3: Collaborative Strategies in 2025
Unified cloud-based data sharing systems
Enables real-time insights, reducing research delays.
Regular interdisciplinary summits and workshops
Encourages knowledge sharing and alignment of research objectives.
AI-bridged workflows
Links laboratory-generated discoveries directly with clinical trials for faster validation.
Cross-trained roles (e.g., research hybrid positions)
Reduces operational bottlenecks through mutual comprehension of workflows.
Future Trends and Scientific Discovery for 2025
Clinical Research Trends
Hyper-Personalization in Medicine:
Greater emphasis on individualized treatments using real-world evidence data.
Decentralized and Hybrid Trials Expansion:
Combining traditional trial setups with remote elements ensures flexibility for global participation.
Regulatory Evolution:
Enhanced FDA/EMA oversight of adaptive clinical trials and expanded consideration of AI-generated insights.
Laboratory Research Trends
Synthetic Biology Boom:
Designing biological systems for novel diagnostic or therapeutic solutions.
Microfluidics Platforms:
Miniaturizing experiments for rapid assay processing and cost efficiency.
AI-Driven Laboratories:
Complete automation of repetitive tasks allows researchers to focus on innovation. Scientists play a vital role in interdisciplinary research teams, contributing significantly to advancing knowledge in laboratory research.
List: Predicted Trends for 2025
AI Integration
Predict recruitment challenges, reduce dropouts, and optimize trial protocols.
Enable predictive simulations for molecular discovery.
Remote Monitoring
Increased use of wearables for real-time patient health tracking.
Real-time integration of IoT devices for instrument feedback automation.
Personalized Medicine Enhancements
Tailoring therapies based on real-time multi-level participant data.
Rapid identification of biomarkers for stratified treatments.
Advanced Automation Systems
Improved reporting speed; fewer manual errors across recorded outcomes.
Reduced operational costs and faster adaptation to experimental demands.
FAQs
What is the main difference between clinical and laboratory research?
Clinical research involves testing interventions directly on humans, while laboratory research focuses on foundational experiments in controlled environments without human participation.
How do technological advancements affect these fields?
Technologies like AI, automation, and wearable devices have bridged gaps, improving efficiency and accelerating discoveries across both domains.
Why is collaboration between clinical and laboratory research important?
Collaboration ensures faster translation of discoveries into treatments, leveraging laboratory insights to guide clinical applications.
What are some future challenges foreseen for 2025?
Data harmonization across platforms, ethical considerations in AI-driven studies, and training interdisciplinary teams remain key challenges.