Research Assistant Role Essentials: Skills & Responsibilities
In today’s clinical, medical, and academic sectors, the demand for qualified research assistants continues to grow. These professionals are the backbone of evidence-driven work—collecting accurate data, maintaining rigorous documentation, and supporting research teams across every phase of a study. Whether it’s in a hospital’s trial unit, a university-backed lab, or a grant-funded study through a Contract Research Organization (CRO), research assistants help transform complex protocols into measurable outcomes. For students and early-career professionals, this role offers a practical gateway into the highly regulated world of clinical research, biomedical development, or academic publication.
Entry into the role is attainable for those with a bachelor’s degree in health sciences, psychology, biology, or related disciplines. More importantly, employers look for hands-on skills: familiarity with clinical trial protocols, data accuracy, and ethical compliance. The research assistant role is not just about administrative support—it’s about being the operational glue of the study. From Institutional Review Board (IRB) coordination to database integrity checks, this position is deeply embedded in both the science and structure of modern research. It’s a career path that rewards attention to detail, adaptability, and continuous learning.
Core Responsibilities of a Research Assistant
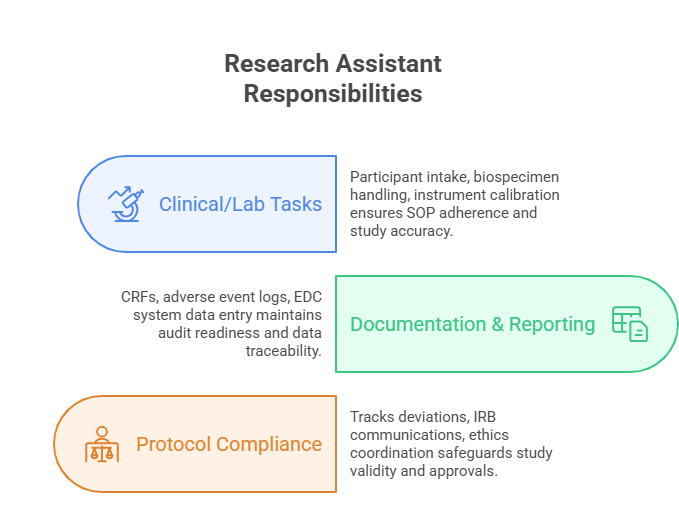
Daily Tasks in Clinical and Lab Settings
Research assistants operate in fast-paced environments where precision directly impacts study integrity. In clinical settings, they often support trial coordinators by preparing informed consent forms, monitoring enrollment logs, and collecting biospecimens. In laboratory environments, responsibilities may include labeling samples, prepping reagents, or calibrating instruments before experiments begin. Across both spaces, accuracy and consistency in following standard operating procedures (SOPs) are non-negotiable.
They also assist in patient interaction workflows—administering questionnaires, explaining study timelines, or guiding participants through visit checklists. Many trials rely on these assistants to be the first point of contact during data collection and monitoring sessions. Their ability to blend scientific awareness with interpersonal tact sets the tone for both compliance and participant retention.
Reporting, Documentation, and Data Entry
Document control is a core pillar of the research assistant’s responsibilities. Each day involves logging study updates, tracking adverse events, and ensuring all entries align with Good Clinical Practice (GCP) guidelines. Using platforms like REDCap, OpenClinica, or Medrio, they enter clinical trial data into Electronic Data Capture (EDC) systems, often double-checking for accuracy.
They're responsible for maintaining Case Report Forms (CRFs), laboratory records, and delegation logs. If even one signature is missing or timestamp incorrect, it can delay approvals or trigger audits. Their role is to reduce friction between research operations and regulatory oversight, ensuring everything is audit-ready.
Protocol Compliance and IRB Communication
Protocol deviations are a major risk to any study. Research assistants play a preventive role by constantly verifying whether on-site activities match approved IRB protocols. When deviations occur—or amendments are made—they draft the necessary correspondence to be submitted to ethics boards. This includes serious adverse event reports, minor amendment notices, and status updates tied to continuing review deadlines.
They also help coordinate IRB submissions for new study startups, including drafting checklists, assembling appendices, and confirming that consent forms are correctly formatted. This backend precision ensures the study’s ethical framework stays intact while minimizing regulatory delays. By doing so, research assistants bridge operational needs with regulatory expectations—a task that requires ongoing vigilance, version control, and deadline tracking.
Must-Have Skills for Research Assistants
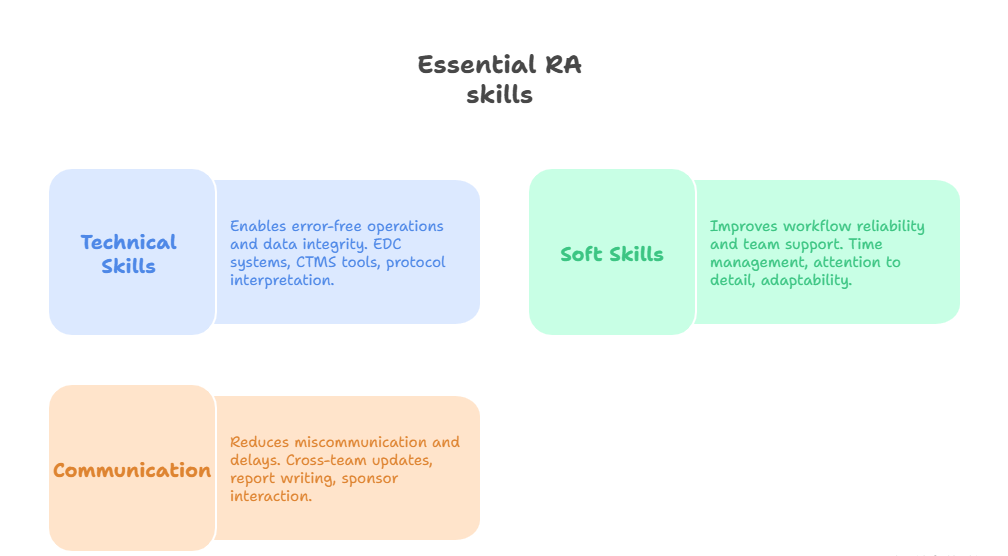
Technical vs Soft Skills Breakdown
Success as a research assistant depends on the right mix of technical proficiency and interpersonal intelligence. On the technical side, employers look for candidates who can interpret protocols, use lab equipment correctly, and handle databases with minimal errors. Whether they're managing blood sample logs or inputting drug reaction data into a clinical trial management system (CTMS), every task demands rigor and consistency.
Soft skills are just as important. Attention to detail helps avoid costly protocol deviations, while time management ensures that participant visits, data entry, and document filing stay aligned with study timelines. Adaptability is essential—research priorities shift often, and assistants must pivot without losing pace. Empathy is also a silent requirement, especially when interacting with patients in vulnerable medical conditions.
EDC Tools, CRFs, and Data Accuracy
A research assistant’s ability to manage data accurately can make or break a study. Familiarity with Electronic Data Capture (EDC) systems is a core requirement. Tools like REDCap, Castor, or Medidata Rave are used to input, clean, and verify trial data. Assistants must understand validation checks, flag discrepancies, and ensure that every Case Report Form (CRF) is 100% audit-ready.
In academic settings, data integrity can influence whether a paper gets published; in clinical trials, it determines if a drug passes review. Research assistants are often tasked with cross-referencing source documents with system entries, ensuring the traceability and reliability of all logged information. This requires an analytical mindset and the ability to follow Good Documentation Practices (GDP).
Team Communication and Study Coordination
No study succeeds without seamless communication, and research assistants serve as liaisons between investigators, coordinators, lab staff, and sponsors. They manage calendars for site visits, set reminders for IRB renewals, and track deliverables to ensure regulatory compliance. In multicenter trials, they often consolidate updates across locations and report on collective progress.
This coordination role also includes prepping agendas for site meetings, circulating SOP updates, and ensuring that all stakeholders are aligned. Assistants who communicate clearly and assertively minimize missteps, especially when handling time-sensitive documentation or protocol amendments. Their clarity keeps workflows moving and reduces the burden on lead investigators or clinical trial managers.
Typical Work Environments and Employers
Hospitals, CROs, and Pharma Companies
Many research assistants begin their careers in hospital-based clinical research units, especially within academic medical centers or teaching hospitals. These environments offer exposure to a wide range of studies—oncology trials, cardiac device assessments, behavioral research—giving assistants firsthand experience with human subjects, regulatory teams, and interdisciplinary collaboration.
Contract Research Organizations (CROs) like ICON, Syneos, or Labcorp Drug Development also employ large numbers of research assistants. These CROs conduct outsourced clinical trials for major sponsors and offer assistants the opportunity to work across multiple therapeutic areas. CRO-based roles often emphasize standardization and high-volume trial execution, which builds procedural expertise fast.
Pharmaceutical companies—especially those with in-house R&D divisions—hire research assistants to support early-phase studies. Their responsibilities may extend to preclinical testing, protocol feasibility, or vendor coordination. These roles typically require a tighter focus on confidentiality, compound tracking, and adherence to internal SOPs written for regulatory submission readiness.
University Research Labs and Grant-Funded Projects
Academic labs are another major employer, particularly for assistants coming from a psychology, neuroscience, or biology background. These positions may involve animal model studies, qualitative surveys, or behavioral experiments. The research assistant supports data collection, manages IRB renewals, and often helps prepare visuals or references for journal publications.
Grant-funded studies, whether NIH- or institution-backed, also rely heavily on research assistants to track budgets, manage timelines, and support reporting deliverables. Since funding is often temporary, these positions can be time-limited—but they provide a rich foundation for graduate school applications or long-term research careers.
Assistants in academic environments often gain broader experience in study design and data interpretation. They may also work closely with principal investigators and graduate students, learning to navigate the early steps of scientific authorship, hypothesis testing, and conference presentation planning.
| Workplace Type | Example Employers | Key Role Features |
|---|---|---|
| Hospitals & CROs | Mayo Clinic, ICON, Labcorp | Research assistants in these environments handle participant visits, biospecimen tracking, and real-time data entry. The work is high-volume and fast-paced, requiring strict adherence to protocols and close interaction with study coordinators and regulatory staff. |
| Pharma Companies | Pfizer, Novartis, GSK | These roles involve early-phase development support, preclinical coordination, and documentation aligned with FDA submission standards. Assistants often help manage lab logistics, digital records, and SOP updates in a secure and highly confidential setting. |
| Academic & Grant Labs | NIH, university research centers | Assistants support study design, participant enrollment, IRB renewals, and manuscript prep. These environments offer exposure to grant writing, data interpretation, and collaborative publishing under the guidance of principal investigators. |
Career Progression and Advancement Paths
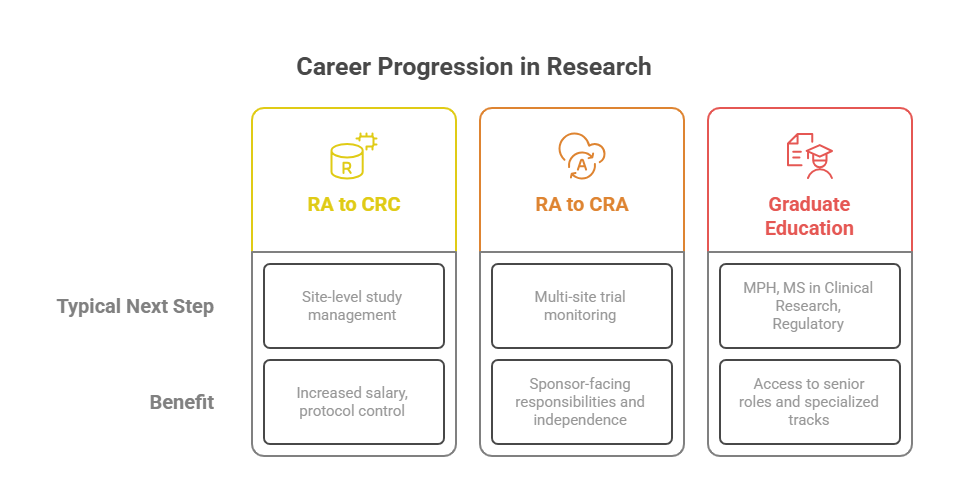
Transitioning to Clinical Research Coordinator or CRA
Many research assistants view the role as a stepping stone to clinical research leadership. With 1–2 years of experience, it’s common to move into a Clinical Research Coordinator (CRC) position. This role involves overseeing entire studies at a site level—managing budgets, participant schedules, and regulatory compliance. CRCs supervise the kind of tasks RAs perform, making prior assistant experience highly relevant.
From there, some professionals pursue Clinical Research Associate (CRA) roles, which focus on monitoring trials across multiple sites. CRAs work for sponsors or CROs and require both independent decision-making and deep regulatory knowledge. Since research assistants already understand EDC systems, GCP guidelines, and source documentation processes, they're well-positioned to excel in remote monitoring or on-site audit preparation roles.
A clear path exists for upward mobility, but it hinges on mastering site-level operations, protocol adherence, and sponsor communication early in one’s RA career.
Certifications and Graduate Program Options
To accelerate career progression, many research assistants pursue certifications like the CCRPS Research Assistant Certification, ACRP’s Clinical Research Coordinator credential, or SOCRA’s Certified Clinical Research Professional (CCRP) exam. These validate hands-on knowledge in ethics, documentation, and site management. Having a credential adds credibility when applying for mid-level or supervisory roles.
Some RAs also apply to graduate programs—master’s degrees in clinical research, public health, or regulatory affairs. These programs offer formal training in study design, biostatistics, and pharmacovigilance, complementing on-the-job experience. Certain programs even waive GRE requirements for professionals with substantial RA backgrounds.
Whether through certification or education, career mobility in research often depends on how quickly an assistant masters documentation, regulatory language, and patient-facing logistics.
Common Challenges Faced on the Job
Data Errors and Site Miscommunication
Even the most experienced research assistants face challenges that can compromise study quality. Data discrepancies are among the most common issues—values entered incorrectly into an EDC system, inconsistent timestamps, or mismatched participant IDs across forms. These errors not only affect the data's credibility but also risk disqualification during audits. Because many studies operate on tight enrollment and reporting timelines, even a small mistake can lead to regulatory hold-ups.
Miscommunication between study sites and sponsors is another critical hurdle. A coordinator may report an update verbally, but if it's not logged and communicated clearly to the sponsor or CRA, protocol deviations can occur. Research assistants are often in charge of cross-functional updates, and any lack of documentation or unclear reporting can snowball into compliance issues.
The solution lies in strict adherence to SOPs, detailed meeting minutes, and the use of centralized tools like CTMS dashboards that sync updates across all stakeholders.
Ethical Dilemmas and Tight Deadlines
Research ethics isn't just about participant safety—it's also about handling ambiguity under pressure. For instance, when a subject forgets to take a study drug, assistants may be asked to note the deviation or recommend next steps. Without proper training, the assistant could underreport the event, risking both ethical violations and data skew.
Tight deadlines present another challenge. Studies often require IRB renewals, sponsor reports, and data locks within days. Research assistants must juggle multiple deadlines at once while keeping documentation error-free and timelines intact. This pressure is magnified during site inspections, where every version of every file must be immediately accessible.
Managing this complexity requires more than efficiency—it demands proactive planning, clear documentation practices, and the confidence to escalate issues when protocols are at risk. Assistants who thrive are those who don’t just execute tasks, but actively prevent bottlenecks and missteps before they occur.
| Challenge Area | Common Issues | How RAs Manage It |
|---|---|---|
| Data Quality | Frequent entry mistakes, mismatched Case Report Forms (CRFs), and invalid timestamps can compromise data integrity and delay regulatory reviews. | Research assistants use real-time validation tools, adhere strictly to SOPs, and perform double-entry checks to ensure data accuracy and audit readiness. |
| Communication Gaps | Unlogged verbal updates, unclear instructions, and missed deadlines often lead to site-level protocol deviations or sponsor dissatisfaction. | Effective use of centralized communication trackers, written meeting summaries, and version-controlled updates minimizes misunderstandings and improves consistency. |
| Ethics & Timelines | Delays in reporting protocol deviations, incomplete IRB renewals, or rushed submissions under pressure can breach compliance. | Assistants rely on calendar alerts, escalation protocols, and early task initiation to ensure timely execution and uphold regulatory ethics. |
How CCRPS Research Assistant Certification Can Help
The Advanced Clinical Research Assistant Certification by CCRPS offers a direct pathway for those seeking structured, expert-level training in clinical research operations. Designed by industry professionals, this program helps early-career candidates and transitioning professionals gain the essential knowledge needed to handle real-world challenges in clinical trials, academic studies, and pharma-sponsored research.
The certification includes over 25 intensive modules, covering topics such as regulatory ethics, documentation standards, data entry protocols, EDC navigation, protocol amendments, and sponsor communication. Every module aligns with ICH-GCP guidelines and global regulatory expectations, ensuring learners don’t just memorize terms—they gain operational fluency in what matters at trial sites.
Learners can complete the program at their own pace, earning 30+ CPD-accredited hours, with downloadable resources, mock assessments, and certification exams. Graduates report improved job performance and faster access to CRC and CRA roles, especially within CROs and research hospitals.
With a credential backed by CCRPS, professionals demonstrate their audit-readiness, protocol fluency, and regulatory literacy—making them more competitive for high-impact research roles where accuracy, compliance, and collaboration are mission-critical.
Frequently Asked Questions
-
Most research assistant roles require at least a bachelor’s degree in a science-related field, such as biology, psychology, public health, or biomedical sciences. Some positions may accept associate degree holders with strong lab experience or certifications. For clinical research roles, having coursework in clinical trial operations, ethics, or statistics adds a competitive edge. Employers also look for practical competencies, including familiarity with EDC systems, Good Clinical Practice (GCP), and basic lab techniques. While not always mandatory, additional training like the Advanced Clinical Research Assistant Certification from CCRPS can dramatically increase job-readiness and credibility for entry-level applicants.
-
In clinical research, research assistant salaries vary by location and employer type. In the U.S., the average annual salary ranges from $42,000 to $60,000, depending on experience, education, and whether you're in a hospital, CRO, or academic setting. Entry-level assistants may earn around $20–$25 per hour, while those with certifications or 2+ years of experience often command higher pay. Assistants working at CROs or pharmaceutical companies tend to earn more than their university counterparts due to the complexity and pace of sponsor-driven trials. Certifications can help move candidates into better-paying CRC or CRA roles faster.
-
Research assistants regularly use Electronic Data Capture (EDC) platforms like REDCap, Medidata Rave, or Castor to input and manage clinical trial data. They may also interact with Clinical Trial Management Systems (CTMS) such as Veeva or Florence eBinders. In academic research, assistants often use Excel, SPSS, or NVivo for data handling and qualitative analysis. Document version control tools, scheduling software, and protocol tracking systems are common in regulated trials. Mastery of these tools is expected in CROs and sponsor settings, and certifications often provide hands-on training in these platforms to boost operational readiness.
-
Yes, many entry-level research assistant positions are designed for graduates or career changers without prior experience. Employers value candidates who show strong foundational skills—attention to detail, data accuracy, and an understanding of research ethics. Enrolling in an online certification program like the Advanced Clinical Research Assistant Certification can bridge the gap by offering exposure to real-world trial processes, documentation standards, and IRB protocols. Volunteer experience, academic research internships, or assisting professors on grant-funded projects also provide valuable experience. Building even small-scale, hands-on involvement in research significantly boosts your chances of getting hired.
-
A research assistant (RA) supports study operations—collecting data, managing documentation, and ensuring compliance with protocols under supervision. They often report to the research coordinator or principal investigator. In contrast, a research coordinator (often a Clinical Research Coordinator or CRC) oversees the entire study site: managing participants, monitoring timelines, and communicating with sponsors and IRBs. While RAs execute tasks, CRCs manage protocols, budgets, and staff, serving as the primary operational lead. Many RAs advance into CRC roles after gaining experience and completing certifications, making it a common internal promotion path in clinical research.
-
The Advanced Clinical Research Assistant Certification from CCRPS equips learners with targeted, employer-relevant skills across ethics, documentation, regulatory processes, and study coordination. Unlike generic online courses, this certification dives deep into clinical trial logistics, from screening patients to handling protocol deviations and IRB communication. It’s CPD-accredited and structured for professionals aiming to work in hospitals, CROs, or sponsor environments. Employers view certification holders as ready-to-train assets, and many CCRPS graduates secure faster placement into CRC, CRA, or regulatory support positions. It signals both initiative and formal proficiency—qualities every research team values.
-
While traditionally an on-site role, especially in clinical trial settings, some research assistant positions have adapted to remote or hybrid models. Data entry, IRB submissions, document tracking, and sponsor communication can all be done remotely if the study infrastructure supports it. Remote roles are more common in academic research, regulatory writing, or post-collection data analysis. CROs and sponsors may also allow hybrid schedules for assistants managing multi-site communications or digital documentation. However, any tasks involving participant interaction, sample handling, or lab procedures usually require in-person presence. Remote-capable RAs still need full regulatory compliance training.
Final Thoughts
Becoming a research assistant isn’t just a job—it’s a strategic entry point into the clinical research field. It demands more than academic credentials; it requires precision, discipline, and a working knowledge of real-world protocols, documentation systems, and ethical compliance. Those who master these fundamentals early often move quickly into higher-paying, leadership-track roles like CRC or CRA.
For aspiring professionals, the Advanced Clinical Research Assistant Certification offers a focused, practical path to readiness. With structured training, CPD hours, and globally aligned modules, it arms candidates with the operational fluency needed to thrive in clinical, academic, or pharmaceutical research environments. Whether you're just starting out or pivoting your career, building the right foundation now opens up a future of long-term growth and industry trust.